ICREA Research Professor, ERC Advanced Grant

Meet Our Scientists Videos
Research information
Background
Colorectal cancer (CRC) is the most frequent and the second-most deadly type of cancer with around 700,000 deaths worldwide. Most colorectal tumors develop as benign lesions but a small proportion progress to more malignant stages when the appropriate alterations in oncogenes and tumour suppressor genes occur. The final and deadliest step in colorectal cancer progression is the metastatic dissemination of CRC cells to foreign organs such as the liver and the lungs.
Research interests
Our lab studies the evolution of intestinal stem cells and their ecosystem during cancer progression, with a particular interests in unraveling how the tumor microenvironment facilitates immune evasion and metastasis. The ultimate goal is to obtain information that help design new therapeutic and diagnostic tools.
Research lines
1. Connecting stem cells to colorectal cancer
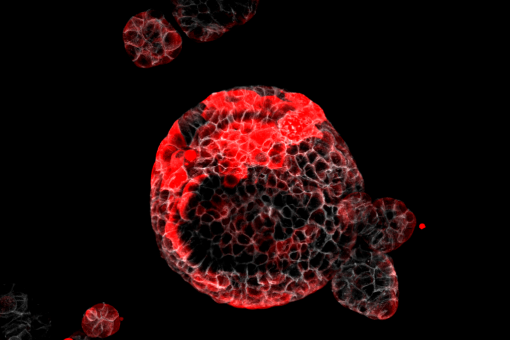
Tissues such as the intestinal epithelium and the hematopoietic system continuously self-renew through the activity of a dedicated population of tissue-specific stem cells. Unlike the bulk of the cells that populate these tissues, adult stem cells are long-lived and generate cellular progeny throughout life to regenerate the multiple specialized, short-lived cells that ultimately perform tissue-specific functions. The cancer stem cell (CSCs) theory states that tumor growth is similarly fueled by small numbers of tumor stem cells hidden in cancers. It explains clinical observations, such as the almost inevitable recurrence of tumors after initially successful chemotherapy and/or radiation therapy, the phenomenon of tumor dormancy, and metastasis. Our laboratory pioneers the notion that colorectal cancers retain a cell hierarchy similar to that of the normal intestinal mucosa. We have characterized extensively tumor stem cells in CRCs and their role in chemotherapy resistance and relapse.
We are currently pursuing the following questions:
- How does the tumor niche specify the CSC state?
- To which extent are tumor-cell phenotypes reversible or interchangeable?
- What type of ‘regenerative’ responses occur upon CSC loss in a tumor, and how are these controlled?
- Which are the intermediate states adopted by tumor cells during invasion?
- How are the metabolic states of tumor stem cells controlled, and what are their effects?
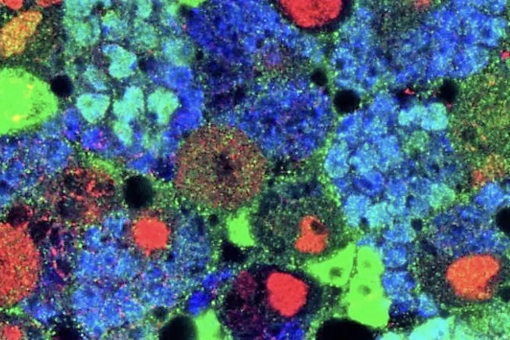
2. Tumor microenvironment, immune evasion and metastasis
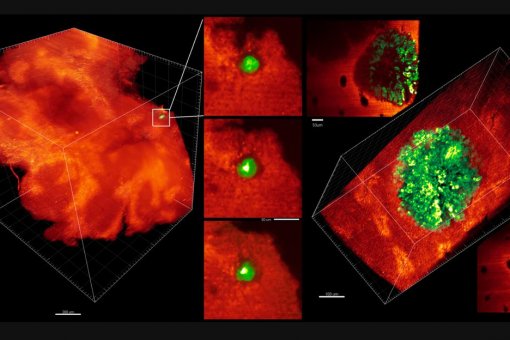
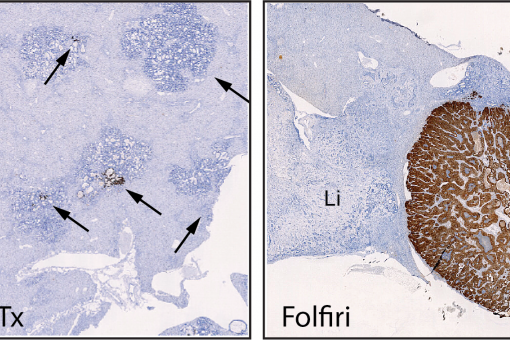
A large proportion of deaths by colorectal cancer are the consequence of metastasis. This process involves the regeneration of a full-blown tumor from disseminated cancer cells and it is intrinsically inefficient mainly due to the inability of isolated tumor cells to colonize host tissues and reinitiate tumor growth in a different environment. The most accepted view is that competences to overcome this bottleneck result from clonal selection of appropriate genetic alterations in cancer cells. However, primary CRCs and metastases do not differ significantly in their mutational content and none of the main alterations in driver oncogenes and tumor suppressor genes correlate significantly with patient outcome.
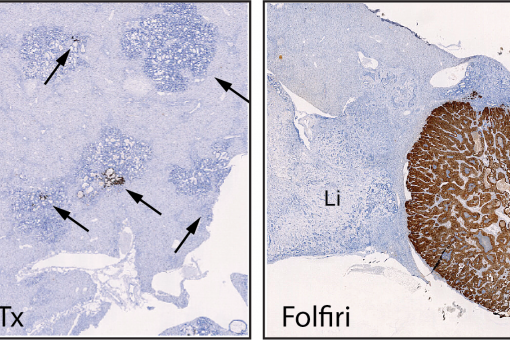
We discovered that metastasis in CRC is a cancer cell non-autonomous process that depends on a TGF-beta-driven gene program expressed in stromal cells. Moreover, we established a pre-clinical model of metastatic CRC in an immunocompetent background and demonstrated that checkpoint immunotherapies are effective against metastatic CRC in the presence of TGF-beta inhibitors This research line holds a great potential to change the clinical management of CRC patients. It also opens up the development of new therapeutic and diagnostic tools for advanced CRC patients based on targeting the TME.
Key questions and research lines in this area include:
- Why some CRCs develop a TGF-beta-activated TME and others don’t?
- How cancer associated fibroblasts mediate the phenomenon of immune-exclusion?
- Genetic analysis of the metastatic niche.
- How systemic factors influence cancer recurrence?
Selected publications
Projects
"A genome editing-based approach to study the stem cell hierarchy of human colorectal cancers" (EditCRC), financiado por el European Research Council (ERC) mediante el Programa de Investigación e Innovación de la Unión Europea 7th Framework Programme. Referencia: 340176

Grup de Recerca consolidat (SGR 2017-2019) de la Secretaria d'Universitats i Recerca del Departament d'Empresa i Coneixement de la Generalitat de Catalunya. Agencia de Gestió d'Ajuts Universitaris i de Recerca (AGAUR). Referencia: 2017 SGR 698


"Abordando el cancer colorrectal desde el microambiente del tumor" financiado por el Ministerio de Ciencia, Innovación y Universidades y por el Fondo Europeo de Desarrollo Regional (FEDER). Referencia: SAF2017-86782-R (FEDER, UE).


"ACRCelerate: Colorectal Cancer Stratified Medicine Network" financiado por la Fundación Científica AECC.

"Dual TGF-beta/checkpoint immunotherapy as curative treatment for microsatellite stable metastatic colorectal cancer: from biomarker to pre-clinical optimization" financiado por la Fundación Científica AECC. Referencia: PROYE18046BATL

"Colorectal Cancer Mouse Tumor Organoids as Pre-clinical Models for Therapeutical Testing" (CRC-MTOs), del European Research Council (ERC) mediante el Programa de Investigación e Innovación de la Unión Europea Horizonte 2020. Referencia: 825836

"Mechanisms of resistance to anti-TGF-beta therapies in metastatic colorectal cancer" financiado porel Worldwide Cancer Research (WCR). Referencia: 19-0005

"Evaluating immunotherapies in a preclinical model of metastatic colorectal cancer" financiado por la Fundación BBVA

"Origin and functions of the tumor stroma in metastatic colorectal cancer" funded by la Fundació La Caixa. Agreement: LCF/PR/HR19/52160018
"Accelerating our ability to understand and target complexity and heterogeneity in cancer through automated imaging of 3D cancer models including patient derived organoids" funded by Fundación Científica AECC. Reference: GEACC19006BAT

“Un nuevo inhibidor del receptor tipo 1 (ALK5) del TGF-beta; para el tratamiento del cáncer metastásico y la fibrosis”, cofinanciado por el Ministerio de Ciencia e Innovación - Agencia Estatal de Investigación (AEI) y por la Unión Europea "NextGenerationEU" mediante el Plan de Recuperación, Transformación y Resiliencia (PRTR). PDC2021-121226-I00/MCIN/AEI/10.13039/501100011033/NextGenerationEU/PRTR

Plan Nacional Spanish Ministry of Science PID2020-119917RB-I00. "Determinantes estructurales y del microambiente en la progresión del cáncer colorrectal y la respuesta a terapia"
![]()
ERC AdvG ERC Advanced Grant. ERC-2019-AdG 884623; ResidualCRC; "ResidualCRC: Residual disease in colorectal cancer: from mechanisms to modelling of therapies that prevent disease relapse"

IMI-Persist H2020-JTI-IMI2-2020_20-04; Persist-Seq; "Building a reproducible single-cell experimental workflow to capture tumour drug persistence Consorcio coordinado por STICHTING ONCODE INSTITUTE"

Súper-resolución con STED FLIM para mejorar ensayos de bioimagen en células vivas y transcriptómica espacial de célula individual en tejidos de animales de experimentación monitorizados in vivo EQC2021-007511-P
Policies
Awards
- 2021 King Jaume I Award in Medical Science
- 2019 City of Barcelona Award
- 2018 Medal from the International Foundation Olof Palme
- 2017 Francisco Cobos Foundation Award to Biomedical Research
- 2016 Carmen y Severo Ochoa Foundation Award
- 2016 Lilly Foundation Award in Pre-clinical studies
- 2015 Life Sciences National Award from Caja Rural de Granada Foundation
- 2014 The Pezcoller foundation-EACR Cancer Researcher Award
- 2013 Joseph Steiner Cancer Research Award (Berna, Switzerland)
- 2013 National Award in Cancer Research “Doctores Diz-Pintado”
- 2010 Banc de Sabadell Award for Research in Biomedicine
- 2006 Debiopharm Life Sciences Award for outstanding research in Oncology – École Polytechnique Fédérale de Lausanne (Lausanne, Switzerland)