Images
Participants

Contact

- Senescent cells, which emerge after tissue injury, create an inflamed aged-like inflamed microenvironment that impairs stem cell function and tissue repair.
- The fatty acid transporter CD36 is highly expressed in senescent muscle stem cells and its inhibition restores the ability of the muscle to regenerate.
- The finding provides a basis for mitigating the loss of muscle regenerative capacity in elderly people and for improving muscle repair in young healthy individuals.
- The study, which was led by the UPF and involved IRB Barcelona, has been published in the journal Nature.
Researchers at the Universitat Pompeu Fabra (UPF), ICREA, CIBERNED, CNIC and Altos Labs, in collaboration with IRB Barcelona and other national and international centres, have described how damaged cells (senescent cells), which inevitably arise after injury, negatively impact tissue regeneration. This mechanism is active in old age, but surprisingly also at young ages. This negative action can be overcome genetically and pharmacologically, thereby restoring the regenerative functions of stem cells.
Tissue regeneration depends on a population of stem cells and neighbouring cells, and the efficacy of this process declines with age. The reasons underlying this decline remain largely unknown.
Work involving mouse experiments led by Dr. Pura Muñoz-Cánoves, ICREA professor at the Department of Medicine and Life Sciences (MELIS) at UPF in Barcelona, CNIC in Madrid, and CIBERNED, and now in Altos Labs San Diego Institute of Science, and Dr. Eusebio Perdiguero (also from MELIS and now in Altos Labs), has revealed that senescent cells exert a novel regulatory function in the muscle regeneration niche by blunting this tissue repair process at all stages of life.
Cellular senescence is a state of irreversible cell cycle arrest that often emerges after tissue damage and in ageing-related diseases. Cells do not die but remain in a state of hibernation. Together with apoptosis (a form of programmed cell death), senescence is one of the mechanisms the body uses to control the unwanted proliferation of tumours. Therefore, the study of these cells is of great biomedical relevance. In addition, senescent cells affect tissue repair processes. In this regard, these cells have been reported to have beneficial effects as tumour suppressors during embryo development, and in liver and skin repair or reprogramming.
However, given the rarity and scarcity of these cells, even in aged tissues, few studies have until now attempted to profile and describe them in vivo.
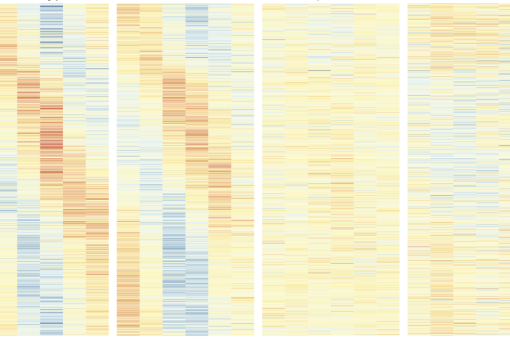
In a study published today in Nature, the team of researchers generated the first transcriptomic atlas of senescent cells of damaged skeletal muscle in mice of distinct ages (“transcriptomic” refers to everything related to RNA or the structures that transcribe the information originally encoded inside the cell nucleus). The scientists found that senescent cells are widely heterogeneous, yet they share common traits, including the secretion of proinflammatory and profibrotic (promoting an excess of fibrous connective tissue) factors. This secretion, in turn, impacts nearby stem cells and hampers their regenerative capacity, thus impairing muscle regeneration. So, it appears that what once was as a good protection tool now turns into a bad one.
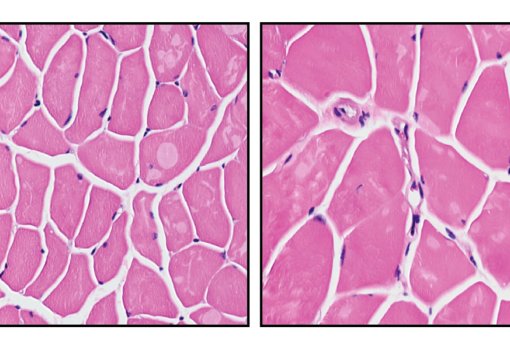
The results showed that reducing the load of senescent cells (either through genetic or pharmacological treatments that induce the death of these cells) improved the regeneration of aged muscles and, unexpectedly, also young muscles. These benefits in young tissue are attributed to decreased inflammation in the stem cell microenvironment, which fosters stem cell functions.
“This is consistent with the notion that senescent cells, even in young tissues, create a hyper-inflamed microenvironment that mirrors inflammation associated with ageing”, says Dr. Muñoz-Cánoves. Thus, senescent cells cause the anticipated ageing of the stem cell niche even in young mice, and so reducing the senescence burden attenuates inflammation of the stem cell niche and enhances muscle repair.
The researchers found that the fatty acid transporter CD36 is highly expressed in these senescent cells and that it is needed for the secretion of these pro-inflammatory signals. “This observation led us to test whether the inhibition of CD36 with blocking antibodies has a pro-regenerative effect in aged muscle,” explains Dr. Salvador Aznar Benitah, ICREA researcher and head of the Stem Cells and Cancer lab at IRB Barcelona. “The results were conclusive and led us to propose that CD36 inhibition could provide a realistic new therapy to boost muscle regeneration in the old and frail,” he adds.
“In addition to the biomedical benefits of targeting senescent cells, the new molecular information provided by the atlas of muscle senescent cells could be used to study the function of senescence in other tissues whose senescent cells have either not been described or in tissues that lack senescent cells,” says Dr. Perdiguero.
The many studies done by several groups demonstrate that senescent cells have diverse effects (beneficial or detrimental) depending on the tissue environment and type, the duration of injury, the persistence of senescent cells, and the age of the organism. Thus, “the role of senescent cells should be studied in distinct contexts, namely in normal, aged and disease states,” says Dr. Muñoz-Cánoves. In this regard, she adds that “taken together, the information presented in this paper will be instrumental for advancing our knowledge of senescent cells and finding new treatments to target them in the context of regenerative medicine and ageing.”
This study has also involved the collaboration of researchers at Kyushu University, (Fukuoka, Japan), Altos Labs San Diego Institute (San Diego, USA), the University of Tokyo (Tokyo, Japan), the Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences (Guangzhou, China), CIC bioGUNE (Derio, Spain), the Institute for Research in Biomedicine (IRB Barcelona), the Luxembourg Centre for Systems Biomedicine (LCSB), and the University of Luxembourg (Luxembourg). The study was funded in part by grants from the European Research Council (ERC), the Spanish Ministry of Science and Innovation, ”la Caixa” Foundation, AFM, MDA, MWRF, and DPP-Spain.
Related article:
Victoria Moiseeva, Andrés Cisneros, Valentina Sica, Oleg Deryagin, Lai Yiwei, Sascha Jung, Eva Andrés, Juan An, Jessica Segalés, Laura Ortet, Vera Lukesova, Giacomo Volpe, Alberto Benguria, Ana Dopazo, Salvador Aznar-Benitah, Yasuteru Urano, Antonio del Sol, Miguel A. Esteban, Yasuyuki Ohkawa, Antonio L. Serrano, Eusebio Perdiguero & Pura Muñoz-Cánoves
Senescence atlas reveals an aged-like inflamed niche that blunts muscle regeneration
Nature (2022) DOI: 10.1038/s41586-022-05535-x
About IRB Barcelona
The Institute for Research in Biomedicine (IRB Barcelona) pursues a society free of disease. To this end, it conducts multidisciplinary research of excellence to cure cancer and other diseases linked to ageing. It establishes technology transfer agreements with the pharmaceutical industry and major hospitals to bring research results closer to society, and organises a range of science outreach activities to engage the public in an open dialogue. IRB Barcelona is an international centre that hosts 400 researchers and more than 30 nationalities. Recognised as a Severo Ochoa Centre of Excellence since 2011, IRB Barcelona is a CERCA centre and member of the Barcelona Institute of Science and Technology (BIST).