Professor (Biochemistry and Molecular Biology Dept. - UB)
Research information
Background
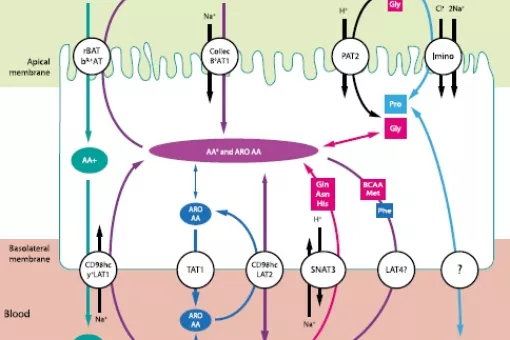
One of the functions of the kidney is the tubular re-absorption of solutes, such as amino acids, filtrated in the glomerulus. Several amino acid transporters are involved in this process and when defective they cause primary inherited aminoacidurias (PIA). Over the last twenty years, studies on PIA have uncovered part of the molecular bases underlying the renal reabsorption of amino acids. The transporters involved in cystinuria, lysinuric protein intolerance, Hartnup disorder, iminoglycinuria, and dicarboxylic amino aciduria have been identified. These studies did not reveal the molecular mechanisms involved in the reabsorption of neutral and acidic amino acid at the basolateral plasma membrane of re-absorptive epithelial cells. Research using mouse models with ablated candidate transporters are expected to fill this knowledge gap.
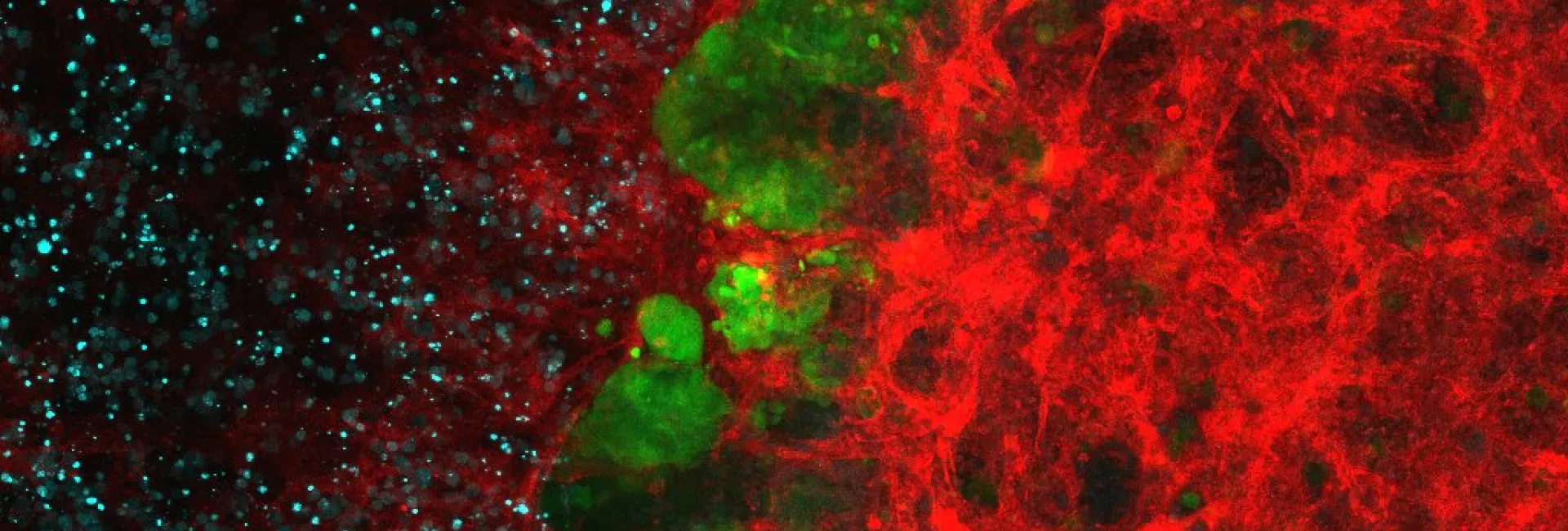
Eleven families of amino acid transporters are coded in the human genome. Transporters from all these families have been associated with disease, whether inherited or acquired. Two of these families of proteins correspond to Heteromeric amino acid transporters (HATs), which are composed of a heavy subunit and a light subunit. 4F2hc (also named CD98hc) and rBAT are the heavy subunits, and eight light subunits are present in human cells. A key feature of these transporters is the dual role of 4F2hc as a partner of six light subunits and as an enhancer of β1/β3 integrin signalling. It is precisely this dual role that makes the transporters 4F2hc/LAT1 and 4F2hc/xCT those most commonly overexpressed in cancer cells.
Research interests
Main objectives of our group are:
- The molecular mechanisms involved in the renal and intestinal re-absorption of amino acids.
- Mechanism of HAT transport at the atomic level.
- Interaction between amino acid transport and integrins.
- Role of transporters in amino acid homeostasis.
Research lines
To achieve our objectives, our group is structured around 4 research lines.
1. Identification of the amino acid transporters involved in the re-absorption of amino acids and those responsible for the development of inherited aminoacidurias.
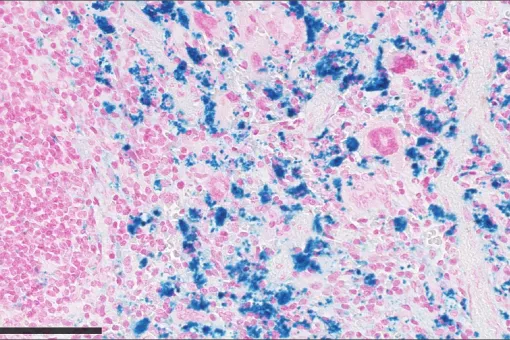
Our laboratory reported the seminal observations that led to the identification of HATs, with the cloning of rBAT and the functional identification of 4F2hc as ancillary proteins of these transporters. Moreover, we have identified five light subunits of HATs (b0,+AT, y+LAT1, y+LAT2, LAT2 and ArpAT). Three of these transporters are involved in renal re-absorption of amino acids: i) System b0,+ (rBAT/b0,+AT), responsible for apical re-absorption of dibasic amino acids and cystine; ii) System y+L (4F2hc/y+LAT1), responsible for the basolateral efflux of dibasic amino acids; and iii) System L (4F2hc/LAT2), responsible for the basolateral efflux of several neutral amino acids, including cysteine. We have also identified the amino acid transporters that are defective in cystinuria (rBAT/b0,+AT) and lysinuric protein intolerance (LPI) (4F2hc/y+LAT1). At present, we are studying the physiology of basolateral transporters that are candidates to play a role in renal and intestinal re-absorption of amino acids. Moreover, we are generating mouse models defective in renal re-absorption-related transporters. Indeed, the b0,+AT null knock-out mouse parallels human cystinuria, including cystinuria lithiasis. This model is currently being used to identify new anti-lithiasic drugs. The conditional y+LAT1 knockout, under study, would mimic human LPI.
2. Structural and structure-function relationship studies of heteromeric amino acid transporters.
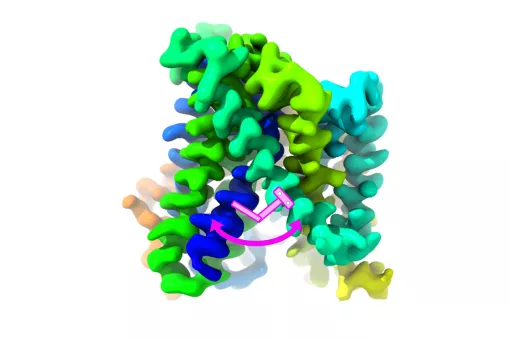
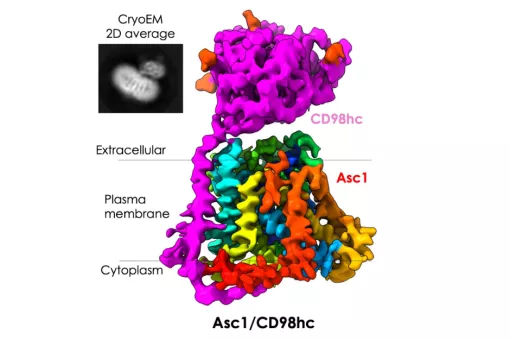
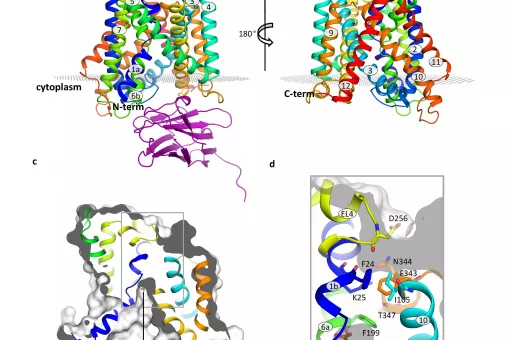
We have demonstrated that the light subunit of HATs is the catalytic part of these transporters. We solved the atomic structure of a bacterial homologue of the light subunits and of the human ectodomain of 4F2hc. We recently obtained the first structural model at low resolution of human HAT, the heterodimer 4F2hc/LAT2. Present goals are to solve the structure of a complete HAT at atomic resolution and delineation of the mechanism of transport using state-of-the-art approaches.
3. Integrins and 4F2hc-associated transporters.
This recently initiated line of research seeks to unravel the cross-talk between integrins and amino acid transport through 4F2hc, a common protein with dual function in transport and integrin signalling.
4. Transporters and amino acid homeostasis.
This relatively new line of research aims to identify the mechanism of transporters involved in the homeostasis of amino acids in the cell. To this end, mouse and cell models with ablated HATs are being studied.
Selected publications
Projects
“Desarrollo de un nuevo fármaco biológico que secuestra la captación de aminoácidos esenciales para administrar específicamente cargas útiles citotóxicas en el tratamiento del cáncer colorrectal” financiado por el Ministerio de Ciencia, Innovación y Universidades mediante la convocatoria del año 2023 de proyectos de colaboración público-privada, del plan estatal de investigación científica, técnica y de innovación 2021-2023. Referencia: CPP2023-010554.

"Amino acid transporter structure to target glutamate transmission in neuro diseases". Fundació La Caixa. Referencia: LCF/PR/HR20/52400017.
“Therapeutical Strategies for cystinuria”. La Marató de TV3. Referencia: 202025-31
“Nueva inmunoterapia contra el cáncer: bloqueo de la reprogramación lipídica basada en el mapeo de transcriptomas de metástasis (LipIMMUNE)”. financiado por MCIN/AEI/10.13039/501100011033 y por la Unión Europea “NextGenerationEU”/PRTR”. Referencia: PLEC2021-007654
"Mecanismos patológicos de los transportadores heteroméricos de amino ácidos", financiado por el Ministerio de Ciencia e Innovación, perteneciente a la Agencia Estatal de Investigación (AEI). Cofinanciado por el Fondo Europeo de Desarrollo Regional. FEDER, una manera de hacer Europa. Referencia: PID2021-122802OB-I00.