Images
MEL-28 ensures that a dividing cell maintains a proper nuclear structure. The work appears in this week's issue of Current Biology.
Every time a cell divides, the protective envelope that surrounds the nucleus is broken down and rebuilt into two new ones. Envelopes are highly complex structures of membranes and proteins which must be precisely reassembled for the nuclei to function.
Scientists at the Institute for Research in Biomedicine (IRB Barcelona) in Barcelona, the European Molecular Biology Laboratory (EMBL) in Heidelberg and the Pasteur Institute in Paris have discovered a protein that plays a crucial role in the assembly and structure of the nucleus. Their work appears in the September 5 issue of Current Biology.
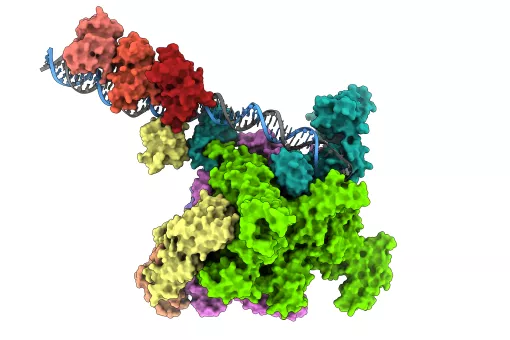
The envelope acts as a barrier between the outer cell compartment, called the cytoplasm, and DNA stored in the cell nucleus. It regulates which molecules are allowed to pass back and forth between the two compartments. Most of this traffic passes through basket-shaped passageways called nuclear pores, which consist of intricately-woven proteins.
“We haven't yet identified all the molecules in the nuclear envelope, and many questions remain about the process by which molecules are granted or denied passage,” says Peter Askjaer of IRB Barcelona.
Oddly-shaped nuclear envelopes are seen in human genetic diseases such as progeria, a rare condition that causes affected children to age prematurely, and some types of muscular dystrophies.
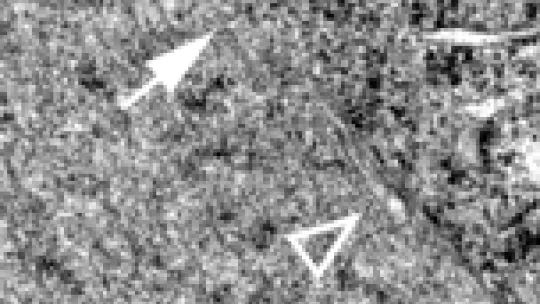
The new study shows that a protein called MEL-28 is a component of nuclear pores in the worm C. elegans, one of biology's most important model organisms. More importantly, it reveals that MEL-28 is one of the key architects as bits of membrane and proteins are drawn together to build new envelopes.
When scientists blocked the activity of MEL-28, they discovered that patches of membranes attached themselves to DNA but couldn't seal themselves off into a complete envelope. A step-by-step analysis showed that without the protein, other molecules are not drawn together properly as envelopes are rebuilt. The components were scrambled; pores were no longer built, and the wrong molecules were able to get access to DNA. Because MEL-28 remains attached to DNA during the entire process of cell division, the scientists believe it plays a crucial role early in the formation of the envelope.
MEL-28 has a close relative in human cells; one of the researchers’ future projects will be to examine whether this molecule plays a similar role in our own species. Oddly-shaped nuclear envelopes are seen in human genetic diseases such as progeria, a rare condition that causes affected children to age prematurely, and some types of muscular dystrophies. “Understanding how the nuclear envelope forms in the first place may eventually help us understand how changes in it can cause these diseases and potentially how they can be treated,” says Askjaer.
About IRB Barcelona
The Institute for Research in Biomedicine (IRB Barcelona) pursues a society free of disease. To this end, it conducts multidisciplinary research of excellence to cure cancer and other diseases linked to ageing. It establishes technology transfer agreements with the pharmaceutical industry and major hospitals to bring research results closer to society, and organises a range of science outreach activities to engage the public in an open dialogue. IRB Barcelona is an international centre that hosts 400 researchers and more than 30 nationalities. Recognised as a Severo Ochoa Centre of Excellence since 2011, IRB Barcelona is a CERCA centre and member of the Barcelona Institute of Science and Technology (BIST).