Organitzat per l'IRB Barcelona en col·laboració amb la Fundació Catalunya La Pedrera.
Presentation

Objectius
El Bojos per la Biomedicina és un curs dirigit als estudiants del primer any de batxillerat que desitgin explorar alguns dels descobriments fascinants que s'estan fent actualment en les ciències de la vida. A través d'aquest curs, els estudiants tindran l'oportunitat d'aprofundir el seu coneixement de la teoria i tècniques científiques en el camp de la biomedicina. Treballaran juntament amb investigadors joves per experimentar com es fa ciència en un institut de recerca internacional, guanyar una mica d'experiència pràctica en les últimes metodologies d'avantguarda i posicionar-se per a una possible carrera professional en les ciències de la vida.
Descripció del curs
Taller d'un any de durada sobre les ciències de la vida per a estudiants de batxillerat. Organitzat per l'IRB Barcelona dins el Programa "Bojos per la Ciència" de la Fundació Catalunya La Pedrera. "Bojos per la Ciència" de la Fundació Catalunya La Pedrera.
Aquest curs combina sessions teòriques i activitats experimentals pràctiques, que es duran a terme durant 16 dissabtes de l'any. El curs tractarà 10 temes científics actuals, que van des de la biologia cel·lular i molecular fins a la biologia estructural i computacional i la química, presentats per investigadors joves de l'IRB Barcelona. En el primer semestre (gener-abril), els tres primers dissabtes es dedicaran a aquestes sessions teòriques generals per a tots els participants. Durant els sis dissabtes següents, es formaran grups petits que entraran als laboratoris per a les sessions pràctiques. A continuació, es repetirà aquest programa amb 5 temes de recerca nous per al segon semestre (maig-novembre). Els estudiants participants s'hauran de comprometre a assistir al curs durant tot l'any.
Els estudiants rebran un certificat de participació en finalitzar el curs en una cerimònia especial de cloenda, a la que podran assistir pares i professors.

Idioma del curs
Totes les xerrades i sessions pràctiques es faran en anglès.
Dates i horaris
El curs tindrà lloc de gener a novembre de 2021, 10.00-14.00h.
1r SEMESTRE
- Div. 15 gener 2021: Inauguració del curs
- Diss. 23 gener: Benvinguda i xerrada 1
- Diss. 30 gener: Xerrades 2-3
- Diss. 6 febrer: Xerrades 4-5
- Diss. 20 febrer: Sessió pràctica 1
- Diss. 6 març: Sessió pràctica 2
- Diss. 20 març: Sessió pràctica 3
- Diss. 10 abril: Sessió pràctica 4
- Diss. 24 abril: Sessió pràctica 5
2n SEMESTRE
- Diss. 22 maig: Xerrada 1
- Diss. 5 juny: Xerrades 2-3
- Diss. 12 juny: Xerrades 4-5
- Diss. 18 setembre: Sessió pràctica 1
- Diss. 2 octubre: Sessió pràctica 2
- Diss. 16 octubre: Sessió pràctica 3
- Diss. 23 octubre: Sessió pràctica 4
- Diss. 6 novembre: Sessió pràctica 5
Preu del curs
Els participants hauran d'abonar a la Fundació Catalunya La Pedrera la quantitat de 330 euros. Consulteu l'oferta de beques al seu lloc web.
Lloc on es realitzarà el curs
Institut de Recerca Biomèdica (IRB Barcelona)
C/ Baldiri Reixac, 10
08028 Barcelona
Qui pot sol·licitar una plaça
Aquest curs està dirigit als estudiants de primer any de batxillerat que tinguin un interès i talent especials en els camps relacionats amb les ciències de la vida.
Els estudiants poden sol·licitar plaça a un màxim de 2 dels programes de la sèrie "Bojos per la Ciència" i finalment només podran participar en un d'ells.
Com sol·licitar una plaça

Les inscripcions s'hauran de realitzar aquí a partir del 16 de setembre 2020.
Els estudiants interessats hauran d'emplenar el formulari de sol·licitud i incloure una carta de motivació. També es demanarà una carta de recomanació directament de dos dels seus professors que coneguin bé l'alumne/a. En el cas de que l'estudiant hagi canviat de centre aquest curs, suggerim que sol·licitin les cartes als antics professors.
La data límit d'inscripció és el 29 d'octubre 2020 (23:59h).
El curs està obert a un total de 25 estudiants. Se seleccionaran els candidats en funció del seu expedient acadèmic, de les recomanacions dels seus professors i de la seva motivació per participar-hi. Es convidarà els candidats preseleccionats a fer entrevistes amb els organitzadors científics al novembre, després de les quals es farà la selecció final. La primera setmana de desembre es comunicarà el resultat als estudiants. Es demanarà als estudiants seleccionats per participar-hi i als seus pares/tutors legals que signin una carta de compromís d'assistir a totes les sessions.
Col·laboradors
Facebook: @LaPedrera.Fundacio
Twitter: @PedreraFundacio
Instagram: #bojosperlaciencia
Facebook: @LaPedrera.Ciencia
Twitter: @PedreraCiencia
Instagram: @lapedrera_ciencia


Per a qualsevol dubte, si us plau contacteu-nos a: irb_outreach@irbbarcelona.org
Dates importants
 29 d'ctubre 2020: Data límit inscripció
29 d'ctubre 2020: Data límit inscripció- 12 de novembre 2020: Contacte amb els candidats pre-seleccionats
- 13-26 de novembre 2020: Entrevistes
- 30 de novembre 2020: Contacte amb els candidats seleccionats
- 15 de gener 2021: Inauguració oficial del curs
- 23 de gener 2021: Inici del curs
- Cerimònia de Clausura - per concretar
Programme
SEMESTER 1
1. Unravelling the Molecular Structure of Life
Blazej Baginski (Structural Characterization of Macromolecular Assemblies)

Proteins and nucleic acids (DNA and RNA) are the basic building blocks of life. They perform a multitude of functions–from sensing, transporting, and enzymatic regulation, to building the cell’s internal skeleton. Therefore, the fold and 3D-structure of these biomolecules is carefully controlled. A protein’s 3D structure determines its activity, creates receptor binding pockets and enzyme active centres.
Crystallography is one of the few methods that allows the structural determination of such macromolecules with atomic precision. By studying the interactions of crystallised molecules by means of high energy X-rays, it is possible to pinpoint the location of atoms and their bonds in a given molecule of interest.
During this course, we will set up a protein crystallisation experiment, learn the operation of high-precision pipetting robots, and cryogenically freeze protein crystals to prepare them for X-ray data collection at the synchrotron.
2. From biomedicine to computational biology


We can recall ourselves as a set of thousands of millions of building blocks that synchronize and match in a perfect way in order to make a living organism. Those building blocks arecalled molecules and comprise four main groups in the living organisms: lipids, carbohydrates, proteins and nucleic acids. Those molecules interact in a perfect way in order to build up a higher level of complexity: cells. As a result, cells are not only the result of molecules but of a perfectly synchronized network of interactions that work extremely efficient in order to make processes such as transcription, translation, mitosis, signalling pathways, etc.
The correct interaction between any two molecules (e.g. a protein and DNA) depends heavily on their 3D structure. In turn, this structure is acquired through a process of folding, guided (again) by intra-molecular interactions. One of our main interests is understanding, modelling, and predicting the 3D structure, dynamics and interactions of nucleic acids.
In this course, we will see biomedicine from a computational perspective. Some of the diseases in the biomedical sector, including cancer, are driven by specific mutations on proteins or nucleic acids which drive an abnormal 3D structure and a lack of native protein to work as expected. Therefore, understanding the dynamics from a bioinformatic, chemistry and physic point-of-view will allow researchers develop therapeutic strategies that might serve as treatments.
3. Identifying the mechanisms regulating expression of linker histones during Drosophila embryogenesis
Srividya Tamirisa (Chromatin Structure and Function)

The eukaryotic genome is tightly packed and organized into a compact nucleoprotein complex called chromatin, made up of DNA and histones. Histones are classified into two types. Core histones form the octamer core of the nucleosome and linker histones connect the linker DNA with the nucleosome. Linker histones play an important role in chromatin structure and function by binding to nucleosomes and modulating accessibility of DNA during replication and transcription. They are known to be de-regulated in several diseases and cancers. In higher organisms there are multiple variants of linker histones with redundant functions. Presence of multiple variants greatly increases the complexity of studying linker histones in vertebrates.
On the other hand, Drosophila melanogaster has only two variants, a somatic variant (dH1) and an embryonic\germline variant (dBigH1), providing an ideal model for studying these proteins. Using Drosophila as a model, we aim to understand the role of linker histones during development and disease. In this course you will learn how linker histones are implicated in development and disease along with practical training in fly genetics, dissection and immunostainings of various Drosophila tissues to look at complimentary expression of dBigh1 and dH1.
4. Manipulating cellular plasticity
Isabel Calvo & Dafni Chondronasiou (Cellular Plasticity and Disease)


The concept of cellular plasticity has gained great relevance during the last years in the context of cancer and tissue repair. Cellular plasticity allows adult cells to regress to stem cell-like states through de-differentiation pathways.
It is possible to convert differentiated cells into pluripotent stem cells (induced pluripotent stem cells or iPSCs) by the simple expression of four transcription factors. These iPSCs are functionally equivalent to embryonic stem cells (ESCs), which are derived from the developing blastocyst and can divide indefinitely while maintaining the capacity to differentiate into any cell type of the organism.
There is an increasing interest in better understanding how these transitions occur both in vitro and in vivo and how they can be manipulated. This knowledge will be directly applied in regenerative medicine to improve current medical treatments.
Students will learn the basic techniques to culture differentiated and pluripotent stem cells and they will be trained to induce the transition between cellular states. Finally, they will have the opportunity to perform in vitro assays that will help us to recognize the ultimate state of pluripotency.
5. "Am I not a fly like them? Or are they not a man like me?" (from "The Fly" by William Blake, 1794)

Maria Victoria Mendiz (Cell Division Laboratory)
The fruit fly Drosophila melanogaster is a well- known organism in scientific research. 100 years of studies have demonstrated how powerful this tiny insect is. Great achievements from the concept of gene inheritance to the basis of normal development or several diseases, including tumorigenesis. The vinegar fly has also helped in so many diverse fields such as behaviour and epigenetics. The simplicity to genetically manipulate the organism offers countless possibilities to study a complex organism.
Despite the obvious differences between humans and Drosophila, it is remarkable that, both at molecular and systemic levels, the fruit fly shares many similarities and conserved pathways with humans. Nearly 75% of human disease-causing genes are believed to have a functional homolog in the fly.
During this workshop, we will learn how scientists work to understand the complex multi-step processes that drive malignant development. We will focus mainly on brain tumors. Brain tumors are among the most catastrophic human cancers due to poor survival rates in patients and there has not been important advances during the last decades. For this reason, brain tumors are such an interesting field to explore.
In this course, you will be introduced to the basis of Drosophila melanogaster research. You will learn about fly genetics through experimentation by crossing flies, identifying genetic markers and balancer chromosomes, and applying other genetic tools used everyday in Drosophila laboratories worldwide. We will learn about the fly anatomy at different stages. In addition, we will also perform in vivo dissections, immunohistochemistry and use advanced microscopy.
SEMESTER 2
1. Strategies to Understand Aging and Senescence
Marta Kovatcheva & Valentina Ramponi (Cellular Plasticity and Disease)


Scientific research and modern medicine have dramatically extended life expectancy, with the average person in the
developed world expected to reach 80 years of age or more. However, this extension in lifespan has had no effect on health span; that is, the number of healthy years a human lives. Aging is still characterised by multiple pathologies including frailty, heart disease, cancer, and neurodegenerative diseases, among many others.
One of the main hallmarks of ageing is cellular senescence, the phenomenon by which normal cells stop dividing. Senescent cells accumulate in an organism over time, secreting pro-inflammatory molecules and contributing to age-related diseases. There is an increasing interest in clinical medicine to better identify and target senescent cells, as their elimination may delay and ameliorate some age-associated diseases.
This course provides hands-on experience using different techniques to induce cellular senescence in normal cells. We will learn state-of-the-art techniques to study the molecular biology of senescent cells, analysing in vitro and in vivo samples. Finally, we will perform classical protocols, such as SAβgal staining, to detect senescent cells. These techniques will help us to understand, identify and target senescent cells for clearance, which is a promising therapeutic approach to extend health span.
2. Alternative Splicing: a source of functional complexity in our genome
Anna Bartomeu (Translational control of cell cycle and differentiation)

Sixty years ago, everyone assumed that a gene was a contiguous sequence of base pairs from which information was transferred to RNA and then to the synthesis of a protein. In 1977, it was discovered that genes of higher organisms are ‘split’ in several well-separated ‘pieces’ along the DNA molecule. These ‘pieces’, termed exons, must be coupled in the process called splicing before the messenger RNA (mRNA) can be translated into a protein. All this is further complicated by the fact that ~95% of human genes are alternatively spliced, encoding two or more protein isoforms.
Alternative splicing is the mechanism by which exons within a single pre-mRNA are differentially joined or skipped to give rise to multiple protein isoforms. This mechanism enables cells to generate immense protein diversity from a limited number of genes.
As a result of recent progress, we now know that a large number of Alternative Splicing changes occur during normal human development and its coordination is crucial for many physiological functions. But it is also starting to be clear that abnormal variations in splicing occur in several diseases, with an important contribution in neurological disorders and cancer. Thus, understanding the regulation of Alternative Splicing is essential to understand the complexity of cell differentiation, organ development and adult tissue homeostasis in health and disease.
In this course, we will use in vitro cell culture systems and look at the current methods that researchers apply to study the regulation of Alternative Splicing.
3. Mirror, Mirror on the Wall… Who Is the Most Helpful Insect of Them All? – Getting to Know Tumorigenesis Through the Fly
Elena Fusari (Development and Growth Control Laboratory)
The fruit fly Drosophila melanogaster has been widely used as a model organism for more than one hundred years to address biological questions in various fields. This organism has emerged as a potent tool for genetic manipulation, offering innumerable possibilities to analyze the detailed interaction between cells and tissues.
One question could be raised: how can a tiny organism such as Drosophila melanogaster be used to understand the pathways of such a complex disease as cancer? Well, the answer is as simple as that: many biological mechanisms are well conserved across evolution, including the ones concerning pathological conditions. This is what allows scientists to translate the knowledge acquired from less complex organisms to more complex ones. For instance, 75% of human disease-related genes and 68% of human cancer-related genes have a counterpart in the fly. We are more similar to the fly than what we may think!
During this semester, we will learn how to study the complex process of tumorigenesis using this model organism, both with a local and systemic approach. We will focus mainly on carcinomas, the most common type of tumor diagnosed in humans.
Carcinomas are derived from epithelial tissue, such as the skin, and they can become invasive or metastatic by spreading beyond the primary tissue layer and surrounding tissues or organs. In aggressive cancer cells, this transition is mediated by the activation of the EMT (Epithelial to Mesenchymal Transition) program, which causes the cells to undergo morphogenetic alterations that increase invasive capacity.
In this course, we will introduce you to the first steps in fly genetics. We will cross flies, identify genetic markers and balancer chromosomes, and apply other genetic tools that we use in the lab every day in order to generate tumorigenic flies and study them. We will also learn about the anatomy of the fly in adult and larvae stages and use advanced microscopy to see several genetic markers in the tumoral tissues. We will also perform in vivo dissections and their respective immunohistochemistry.
4. CRISPR WARS: The Gene Strikes Backs
Luis Povoas (Gene Translation Laboratory)
 Some years ago, during the process of constructing a fly model to study human diseases our lab found a very interesting protein that seems to be responsible for many different processes in the cell. This protein is a derivate of a group of proteins responsible to introduce amino acids in tRNA, to enable protein synthesis. We called it SLIMP (seryl-tRNA synthetase-like insect mitochondrial protein). Past studies suggest that this new protein has acquired an essential function in insects, being one of the most interesting results obtained so far, the role of SLIMP in cell cycle progression. To uncover more of SLIMP’s functions, our lab is using a revolutionary technology that changed the way we make science. This technology is the CRISPR editing system. This tool enables us to precisely edit the genome of live cells. With it, we can remove genes (knock-out), introduce genes (knock-in), introduce markers to follow a specific protein (tags) and others. Therefore, with this technology, we expect to uncover SLIMP intracellular location, protein-protein interactions and try to understand the SLIMP knockdown phenotypes in relation to DNA damage and cell cycle checkpoints. During this workshop, you will be introduced to concepts of molecular cloning, cell culture and CRISPR technology. May the science be with you!
Some years ago, during the process of constructing a fly model to study human diseases our lab found a very interesting protein that seems to be responsible for many different processes in the cell. This protein is a derivate of a group of proteins responsible to introduce amino acids in tRNA, to enable protein synthesis. We called it SLIMP (seryl-tRNA synthetase-like insect mitochondrial protein). Past studies suggest that this new protein has acquired an essential function in insects, being one of the most interesting results obtained so far, the role of SLIMP in cell cycle progression. To uncover more of SLIMP’s functions, our lab is using a revolutionary technology that changed the way we make science. This technology is the CRISPR editing system. This tool enables us to precisely edit the genome of live cells. With it, we can remove genes (knock-out), introduce genes (knock-in), introduce markers to follow a specific protein (tags) and others. Therefore, with this technology, we expect to uncover SLIMP intracellular location, protein-protein interactions and try to understand the SLIMP knockdown phenotypes in relation to DNA damage and cell cycle checkpoints. During this workshop, you will be introduced to concepts of molecular cloning, cell culture and CRISPR technology. May the science be with you!
5. From biomedicine to computational biology (Part II)
Alba Sala (Molecular Modelling and Bioinformatics)

The interaction between small molecules is essential to living organisms. Amongst these molecules we find protein and nucleic acids, whose interaction plays a crucial role in biology (e.g., in the storage, repair, expression and regulation of genetic information).
Protein-DNA interaction is mostly defined by two mechanisms: what is called a direct or base read out and an indirect or shape readout. The former describes the ability of proteins to distinguish different binding modes. The later, focuses on the importance of certain protein and DNA sequences to adopt different 3D structures which determine the binding sites. The amount of importance given to these two mechanisms varies from protein to protein, but it is crucial to understand the role of a protein’s 3D structure.
In this course we will study the 3D structure of different proteins from a computational point of view. A protein’s structure is believed to dictate its function, and once a protein’s shape is understood, its role within the essential biological mechanisms can be determined; giving then information to scientists to develop new methodologies that work with a protein’s shape.
Venue
El curs de Bojos per la Biomedicina tindrà lloc a les instal·lacions de l'IRB Barcelona.
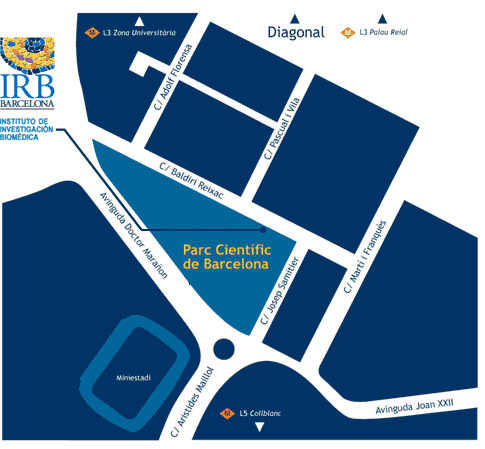
Institute for Research in Biomedicine (IRB Barcelona)
Parc Cientific de Barcelona
C/ Baldiri Reixac, 10
08028 Barcelona

