Meet Our Scientists Videos
Research information
Background
The unifying concept that has guided our research over the years is that tumor suppressor genes protect from many types of damage regardless of the particular detrimental consequences of the damage. In other words, tumor suppressors protect from damage even if that damage is not going to produce cancer, but a degenerative disease. According to this view, cancer protection is just one of the outcomes of tumor suppressors, being other outcomes protection from chronic diseases, from nutritional overload, from tissue injuries, or from aging.
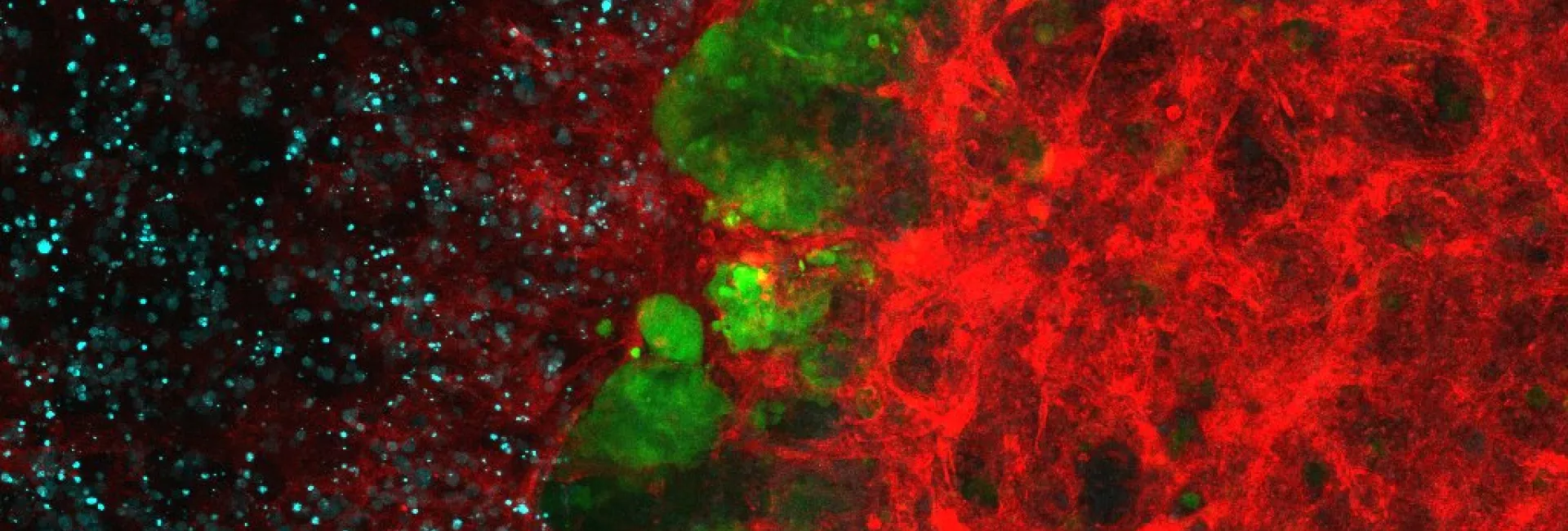
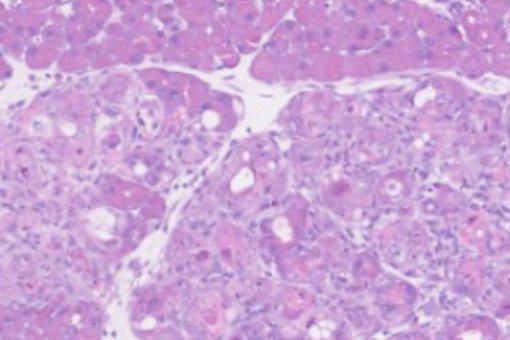
Tumor suppressors often trigger a stereotypic cellular state known as cellular senescence, and our group has made seminal contributions to the understanding of cellular senescence from a physiological perspective.
During the last 5 years, we have made key contributions to the advance of our understanding of tumor suppression, damage, cellular senescence and tissue regeneration:
- The primary function of cellular senescence is to orchestrate tissue regeneration (Cell 2013; Science 2016).
- Tumor suppressors protect from aging (Cell Metab. 2012) and from the damage caused by nutritional overload, including obesity and metabolic syndrome (Cell Metab. 2015).
- Tumor suppressors regulate cell plasticity (Nature 2009; Cell Stem Cell 2012).
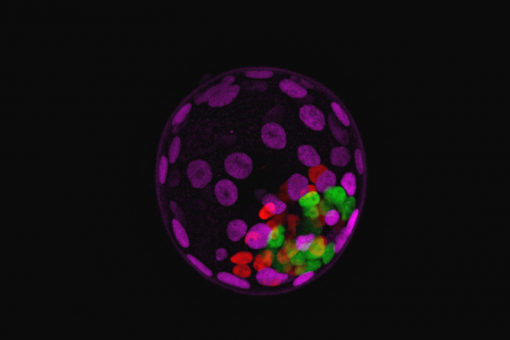
- Cell plasticity can be induced and manipulated in vivo (Nature 2013).
The key emerging paradigm is that tumor suppressors, by triggering cellular senescence, recruit inflammatory cells and create a tissue microenvironment that favors tissue repair and regeneration.
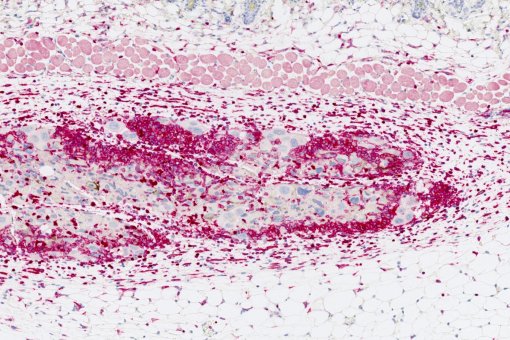
We are dissecting the molecular mechanisms and we are applying this knowledge to the treatment of various diseases, including pulmonary fibrosis and cancer.
Research lines
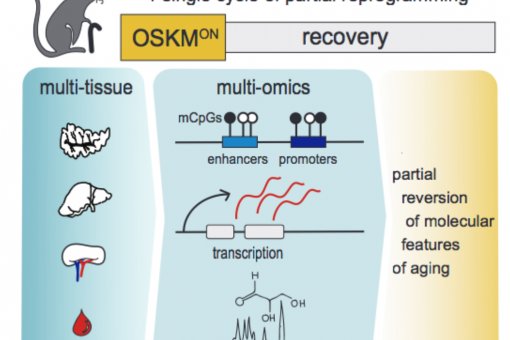
1. Tissue regeneration in the reprogrammable mice.
We are actively studying tissue regeneration in our reprogrammable mice (where we can induce the four Yamanaka factors in vivo) and how this is affected by tissue injury, senescence and inflammation.
2. Therapeutic effects of elimination of pathological senescent cells.
We have a very original project on the use of silica nanoparticles to deliver drugs selectively into senescent cells. We are focused on their therapeutic potential in pulmonary fibrosis.
3. Manipulating and understanding pluripotency.
We have several projects aimed to manipulate and stabilize pluripotency with chemical compounds, both in mouse and in human cells. For example, we can hyperactivate the Mediator complex with a chemical compound and in this manner we can stabilize the naïve state of pluripotency in mouse and human cells. We are attempting to deliver reprogramming chemicals in vivo to enhance tissue regeneration.
4. Targeting pluripotency in cancer.
We have a strong line of research on cancer and in this regard we have identified new chemical compounds that selectively target cancer stem cells.
5. Understanding aging.
We have several projects aimed to understand the connection between metabolic pathways, tumor suppressors and aging.
Selected publications
Projects
“New Frontiers in Cellular Reprogramming: Exploiting Cellular Plasticity” (CELLPLASTICITY), financiado por el European Research Council (ERC) mediante el Programa de Investigación e Innovación de la Unión Europea Horizonte 2020. Referencia: 66962.

"Joint Training and Research Program on Lifespan Regulation Mechanisms in Health and Disease" (HealthAge), financiado por el Programa de Innovación e Investigación de la Unión Europea Horizonte 2020 bajo la acción Maire Sklodowska-Curie (ITN). Referencia: 812830.
"Senescencia y plasticidad celulares: función en reparacion tisular y en enfermedad" (REPAIR), cofinanciado por el Ministerio de Ciencia, Innovación y Universidades- Agencia Estatal de Investigacióny por el Fondo Europeo de Desarrollo Regional (FEDER) de la Unión Europea. Referencia: SAF2017-82613-R.



"Desarrollo preclínico de anticuerpos inmunomoduladores ANTI-PD-L2 para el tratamiento de patologías inducidas por el daño celular - validación de la estrategia en tumores residuales en fibrosis ” (IMMOPDL2), cofinanciado por el Ministerio de Ciencia, Innovación y Universidades- Agencia Estatal de Investigación y por el Fondo Europeo de Desarrollo Regional (FEDER) de la Unión Europea (Objetivo temático: Promover el desarrollo tecnológico, la innovación y una investigación de calidad). Proyecto Retos – Colaboración. Referencia: RTC-2017-6123-1.



Grup de Recerca consolidat (SGR 2017-2019) de la Secretaria d'Universitats i Recerca del Departament d'Empresa i Coneixement de la Generalitat de Catalunya. Agencia de Gestió d'Ajuts Universitaris i de Recerca (AGAUR). Referencia: 2017 SGR 282.


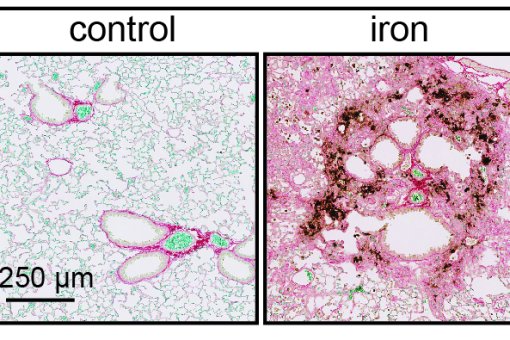
"Iron as a driver of fibrosis and regeneration" (IRONAGE), financiado por el Programa de Innovación e Investigación de la Unión Europea Horizonte 2020 bajo la acción Maire Sklodowska-Curie (IF). Referencia: 794744.
"Cancer and ageing-associated diseases: new frontiers and new strategies" (CANCERAGE), cofinanciado por el Ministerio de Economía y Competitividad y por el Fondo Europeo de Desarrollo Regional (FEDER) de la Unión Europea. Referencia: SAF2013-48256-R (MINECO/FEDER, UE.


"Cell Senescence: Mechanisms and Therapies" (SENESTHERAPY-III), financiado por el Ministerio de Ciencia, Innovación y Universidades- Agencia Estatal de Investigación. Referencia: RED2018-102698-T.


“Desarrollo preclínico de un nuevo activo modulador de la fibrosis y la senescencia para el tratamiento de la enfermedad de hígado graso no-alcohólico” (HEPATOSEN), financiado por el Ministerio de Ciencia e Innovación - Agencia Estatal de Investigación y el Fondo Europeo de Desarrollo Regional de la Unión Europea (FEDER, UE). Retos – Colaboración 2019. Referencia: RTC2019- 007125-1



“Novel senolytic drugs for the treatment of lung and kidney fibrosis - SENFIB”, financiado por el European Research Council (ERC) mediante el Programa de Investigación e Innovación de la Unión Europea Horizonte 2020. Referencia: 963988.
Suport obtingut de l’FSE a través de les Ajuts per a la contractació de personal investigador novell (FI). El Fons Social Europeu dona suport a la creació d’ocupació, ajuda a las persones a aconseguir millors llocs de treball i garanteix oportunitats laborals més justes per a la ciutadania de la Unió Europea.