Images
Joan Roig’s team collect three-dimensional information of protein complexes involved in cell division.
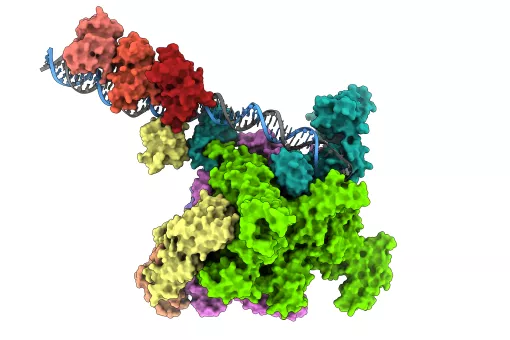
Collaboration between researchers at the Institut de Biotecnologia i de Biomedicina (IBB) of the “Universitat Autònoma de Barcelona”, the Institute for Biocomputation and Physics of Complex Systems in Zaragoza, and IRB Barcelona has led to the publication of the first scientific paper on data collected from the BL13-XALOC beamline of the Alba Synchrotron, located in Cerdanyola de Vallès, a facility that which allows scientists to obtain 3D structures of macromolecular complexes.
The team at IRB Barcelona headed by the research associate Joan Roig, who works in Jens Lüders “Microtubule Organisation” group, in collaboration with David Reverter’s lab at IBB, has resolved two structures that shed light on the regulation of certain proteins involved in correct cell division.
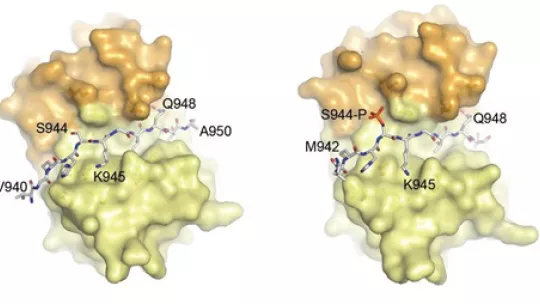
In particular, the researchers have managed to achieve 3D information of the interaction between the protein LC8 and two fragments of Nek9, one of them modified by phosphorylation. Roig has previously demonstrated that LC8 controls the binding of the signaling protein Nek9 with the related molecules Nek6/7. Nek9, Nek 6 and Nek 7 are a set of relevant proteins for the correct separation of chromosomes during cell division and they ensure that each daughter cell receives the appropriate genetic material. The new study provides insight into the regulation of the signaling cascade involved in cell division through this set of proteins. “This work is helping us to understand how the different proteins we study bind and interact and how this affects their regulation and physiological function. This kind of information could be highly relevant in the therapeutic strategies seeking to target Neks”, explains Joan Roig.
Reference article:
Structural analysis of the regulation of the DYNLL/LC8 binding to Nek9 by phosphorylation
Pablo Gallego, Adrian Velazquez-Campoy, Laura Regué, Joan Roig and David Reverter
Journal of Biological Chemistry (2013) doi: 10.1074/jbc.M113.459149 jbc.M113.459149.
Link to news from Alba Synchrotron
About IRB Barcelona
The Institute for Research in Biomedicine (IRB Barcelona) pursues a society free of disease. To this end, it conducts multidisciplinary research of excellence to cure cancer and other diseases linked to ageing. It establishes technology transfer agreements with the pharmaceutical industry and major hospitals to bring research results closer to society, and organises a range of science outreach activities to engage the public in an open dialogue. IRB Barcelona is an international centre that hosts 400 researchers and more than 30 nationalities. Recognised as a Severo Ochoa Centre of Excellence since 2011, IRB Barcelona is a CERCA centre and member of the Barcelona Institute of Science and Technology (BIST).