The Metastasis Challenge is an IRB Barcelona initiative that calls society to action in the fight against metastasis. We want to appeal to citizens, organisations, and companies to participate in raising 5 million euros, which will allow us to accelerate research, recruit new talent, purchase cutting-edge technology and equip new laboratories.
Despite breakthroughs in cancer research in recent years, metastasis continues to be responsible for 90% of cancer-related deaths. Metastasis is the spread of cancer to different organs in the body. While we know quite a lot about tumours and how to treat them, there is still a long way to go before we will be able to diagnose, prevent and cure metastasis.
Watch the video below to find out about the advances we have made in metastasis research
What is metastasis
1. What is metastasis?
Cancer is a disease caused by errors, called “mutations”, in the genetic material (that is, in DNA) of our cells. If our body is not able to correct these mutations, they accumulate, causing cells to multiply in an uncontrolled manner and give rise to tumours.
Metastasis is the spread of cancer from the primary tumour (original tumour) to other vital organs, such as the brain, lung or liver, through blood or lymph vessels. Primary tumours are seldom lethal. In contrast, metastatic tumours (also called metastases) are difficult to control
The word “metastasis” is used in the context of solid tumours, that is to say, those that do not arise from blood cells. Those that arise from such cells are called “liquid tumours, the most common of which are leukaemias and lymphomas.
In recent decades, research focused on the initial stage of the disease (primary tumour) has led to a significant improvement in patient survival. Currently, thanks to advances in surgical techniques, treatments and early detection protocols, 60% of cancers are cured or become chronic conditions.
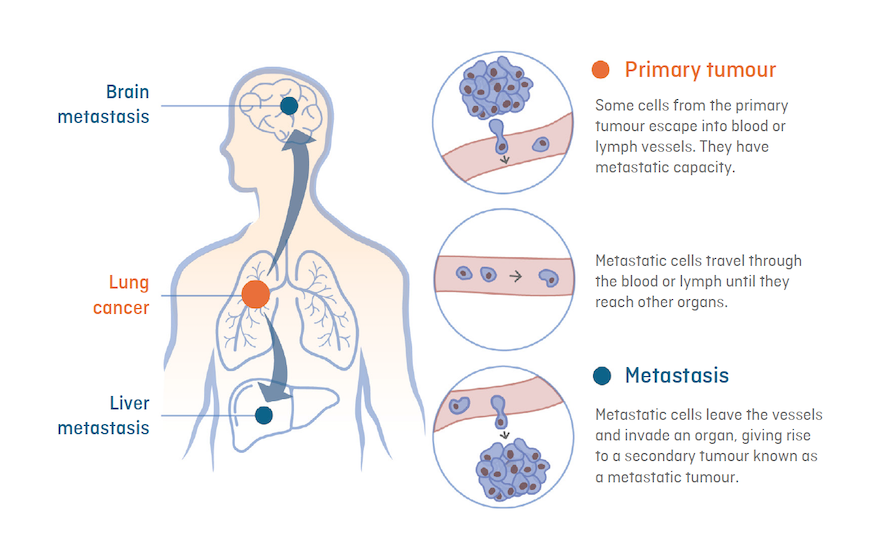
Unfortunately, metastatic tumours do not behave like primary tumours, which makes available therapies ineffective in treating most patients who suffer from them. Therefore, it is urgent to devise new strategies for the prevention, diagnosis and treatment of metastasis.
2. How does metastasis develop?
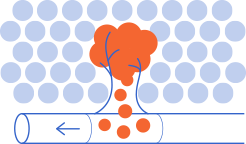
Metastasis arises from cancer cells that can migrate from the primary tumour and reach another organ, where they settle and give rise to a new tumour (a metastasis or metastatic tumour).
For this to occur, these cells must “travel” in four phases:
- Leave the primary tumour.
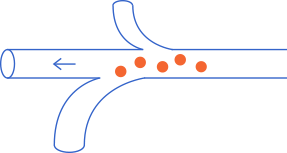
- enter the blood or lymphatic stream and survive while they circulate through the body.
- Leave the circulation and colonize and adapt to the conditions of the organ they reach, which are very different from those of their organ of origin. This “journey” is known as the “metastatic cascade””.
Most cells that escape from the tumour are not able to survive in the blood or lymphatic stream. Only those cells that acquire mutations (changes in genetic material or DNA) advantageous for surviving outside the tumour are capable of colonizing other organs. Said mutations provide the cells with abilities such as a low multiplication rate (which confers resistance to chemotherapy) or the capacity to go undetected by the immune system.
From a therapeutic perspective, understanding how cancer cells acquire the capacity to leave the primary tumour and “travel” is important to prevent metastasis in patients diagnosed with primary tumours, while understanding the characteristics acquired by cells that reach the secondary organ (successful colonization) can lead to the development of effective therapies for patients who already have metastasis.

3. What is cancer relapse?
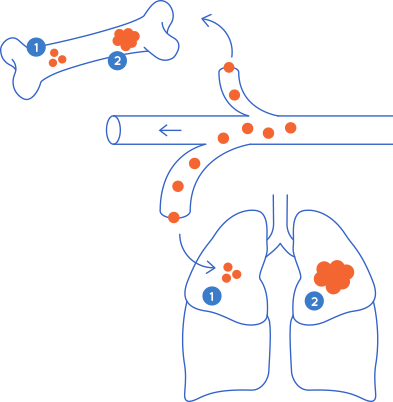
Relapse (also known as “recurrence”) is the reappearance of cancer after the primary tumour has been removed by surgery or chemotherapy and when the disease has been undetectable for a certain period. Relapse can be local (when the tumour grows back at the primary site) or, more commonly, in the form of metastases (the tumour grows in a distant organ). One factor that explains relapse is that the cells initiating metastasis can enter a state of dormancy in which they do not multiply. This state makes them resistant to chemotherapy, which attacks mainly proliferating cells.
Metastatic cells go into “hibernation” (or a dormant state) because the physical, metabolic (availability of nutrients, oxygen, etc.) and immune conditions of the colonized organ (host organ) hinder their multiplication. They remain in this state until the host organ environment is favourable to generate a new tumour.
In some cases, tumour cells send signals to the resident cells of the host organ to generate favourable conditions, such as the creation of new blood vessels, which carry nutrients and oxygen for tumour growth. Metastatic cells can also undergo mutations (changes in the genetic material or DNA) that confer beneficial characteristics for survival, such as the capacity to become invisible to the immune system and thus avoid detection and elimination
In most cases, metastatic cells can remain dormant for months, years, or even decades, before forming a clinically detectable metastatic tumour. Some primary tumours are more likely to develop metastases than others (column “metastatic relapse rate” of the table below), and the time these metastases take to appear varies depending on the organ in which the primary tumour originates (column “latency period” of the table below):
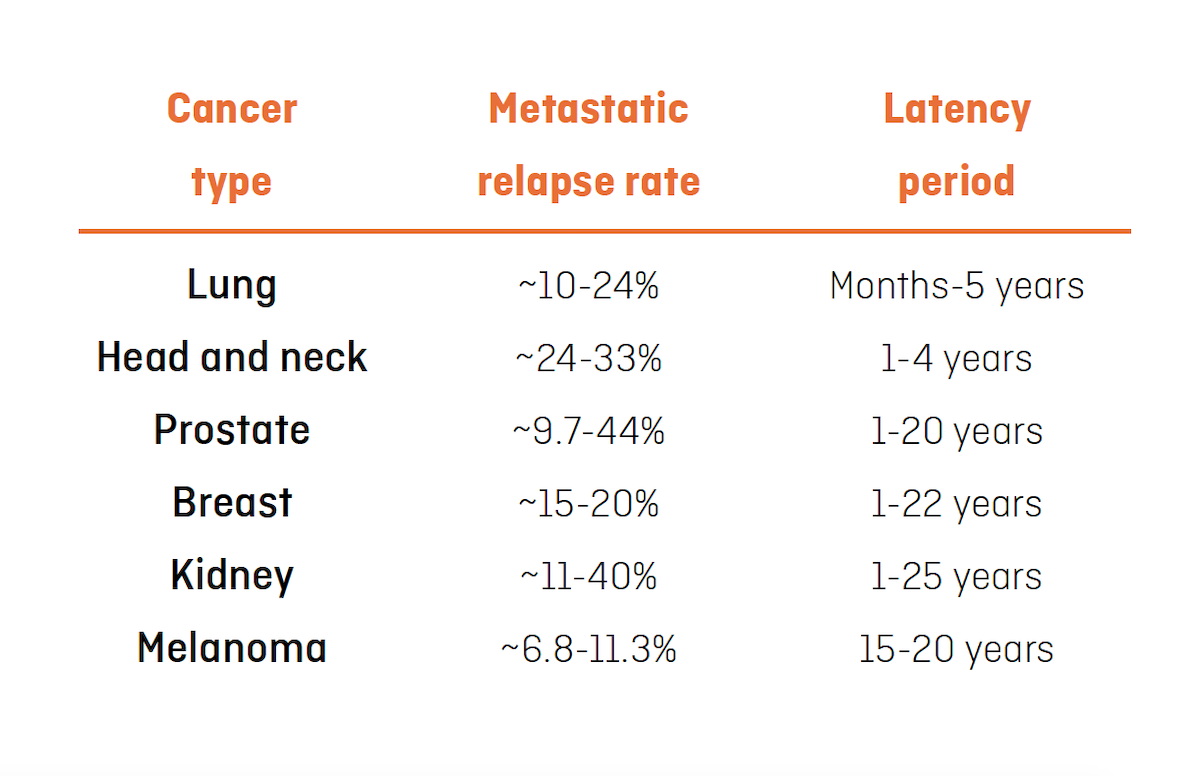
The table above shows metastatic relapse rate and latency period in patients with different types of cancer. The metastatic relapse rate refers to the risk of having a relapse after removal of the primary tumour and the latency period refers to the period in which relapse can occur. Table adapted from Kim Márquez-Palencia & Malladi, Frente. Inmunol. (2019).
4. Which organs do tumours form metastases in?
Metastasis will develop in a specific organ depending on the tissue in which the cancer has originated. Certain types of cancer are more likely to spread to specific organs, a process called "metastatic organotropism". For example, colon cancer usually metastasizes to the liver because the circuit followed by the blood that drains the intestines flows to the liver through the hepatic portal vein. Once a metastatic tumour is generated in the liver, a secondary metastatic tumour can arise in the lungs, a process known as “sequential metastasis”.
Cells from the same type of cancer may be more likely to colonize some organs or others depending on their properties. An example is the different subtypes of breast cancer: estrogen receptor-positive (ER+) tumours are more likely to metastasize to bone, while ER- tumours tend to go to organs such as the lungs, liver or brain.
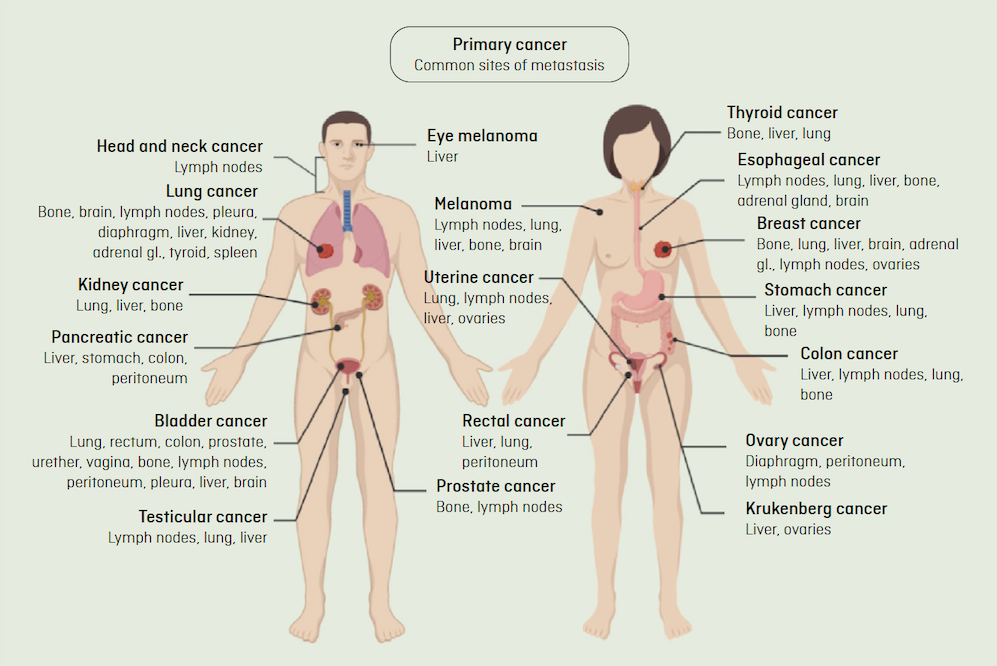
The picture above de dalt shows the common routes of metastasis. Clinical observations suggest that most cancers metastasize to specific organs, a process known as "metastatic organotropism." Adapted from Fares J. et al, Nature, 2020.
5. How is metastasis detected?
In the early stages of metastasis, only a small number of cells survive. In most cases, these are isolated cells or cells found in small groups (“micrometastases”), which makes their detection by conventional imaging methods, such as positron emission tomography (PET) or magnetic resonance imaging (MRI) very difficult.
Early detection methods aim to find tumour cells before they reach the organ where they will metastasize. A very widespread practice is the exhaustive analysis of the lymph node closest to the primary tumour, called the “sentinel node”, to which the cancer is likely to spread first. When tumour cells are present, this node is usually removed, thus establishing a “firewall”.
Other methods seek to detect the trace of tumour cells in circulation through the analysis of blood samples (liquid biopsies). This can be performed by detecting cancer-specific signals (biomarkers) on the surface of tumour cells, or by detecting genetic material (DNA) or circulating vesicles (exosomes) from tumour cells.
In both cases, the low number of cells present in the early stages of metastasis continues to be the main obstacle to the reliable diagnosis of the disease. For this reason, in recent years, considerable efforts are being channelled into describing metastasis-initiating cells and improving the sensitivity of diagnostic methods.
6. Why is it so difficult to treat metastasis?
Most cancer medications are ineffective in treating metastasis, often because metastatic cells acquire resistance to therapy. Immunotherapy (a technique that strengthens the cells in the patient's immune system) is emerging as a revolutionary strategy for the remission or chronification of some types of advanced cancer. However, unfortunately, there are still no effective treatments for most metastatic cancers.
Often, metastasis treatments seek to stop the growth of metastatic tumours and thus prevent the progression of the disease and turn it into a chronic condition. If metastatic cancer responds well to treatment, some people can live for years after the initial diagnosis of metastasis.
The factors that contribute to the difficulty in eliminating metastatic tumours are as follows:
- In many cases, metastases develop in vital organs, such as the lungs or brain, which generally makes them inoperable, as it would affect the functioning of the affected organ and put the patient's life at risk.
- The characteristics of metastatic cells differ from those of the primary tumour cells, and, in certain cases, confer resistance to treatments to which the latter were sensitive1. These characteristics, which are usually advantageous for the survival of metastatic cells, are acquired from mutations that accumulate in DNA.
- Micrometastases are formed by one or several cells that, in many cases, are “sleeping” (dormant) until they have favourable conditions in which to multiply. These are individual cells or small groups of cells that are difficult to detect by conventional imaging techniques (positron emission tomography (PET), magnetic resonance imaging (MRI), etc.
One of the current challenges faced by biomedical research is the development of methods for the detection of micrometastases, as well as a greater understanding of the weaknesses of metastatic cells, which will enable us to design therapeutic strategies for their elimination.
7. What do we do at IRB Barcelona to beat metastasis?
Metastatic tumours do not behave like primary tumours, nor do they normally respond to the same treatments, which makes metastatic cancer an extremely complex disease. At IRB Barcelona, we focus our multidisciplinary experience on studying metastasis and developing innovative therapeutic strategies.
Our objective is to contribute to answering three fundamental questions:
- How can the risk of relapse be predicted?
- How can metastasis be prevented?
- How can metastasis be treated?
At IRB Barcelona, we have 30 research groups that bring together more than 450 scientists specializing in different disciplines. Our laboratories are made up of multidisciplinary teams, where biologists, chemists, computer scientists or doctors work with the most advanced technology in microscopy, genome sequencing, bioinformatics, and the synthesis of chemical compounds.
Thanks to this combination of expertise, we have the opportunity to discover new strategies for the diagnosis, prevention and treatment of metastasis.

Below are some examples of our research results:
A new tool to roll out personalized cancer medicine
During tumour growth, cancer cells accumulate alterations (mutations) in their genome. The Biomedical Genomics laboratory, led by Dr. Núria López-Bigas, has developed a computational tool (the “Cancer Genome Interpreter”, CGI) based on machine learning that allows the identification of the mutations that are important for the progression of the disease and those that may be related to the treatment response.
CGI seeks to help medical personnel prescribe the most appropriate treatment for each patient. Currently, IRB Barcelona collaborates with different entities across Europe (patient associations, hospitals, and other research centres), to implement this personalized medicine tool in health systems.

A test to predict the risk of bone metastasis in breast cancer patients
Approximately 1 in 8 women will develop breast cancer during their lifetimes, and in 15% of these cases, the cancer will spread to the bones. Although breast cancer has a high survival rate (about 85%), the prognosis worsens considerably in cases with metastasis.
The Growth Control and Cancer Metastasis research group, led by Dr. Roger Gomis, has identified a gene (MAF) that is key in the development and, more specifically, in the spread of cancer cells to bone. Based on this discovery, the company Inbiomotion, a spin-off from IRB Barcelona and ICREA founded by Dr. Gomis, has developed a test (MAF test®) that predicts the probability that a breast cancer patient has of developing bone metastases years after having had the disease, thus determining whether this person could benefit from adjuvant (booster) chemotherapy treatment to prevent relapse. Work is now underway to ensure that the MAF test® is incorporated into clinical practice and is commonly used in hospitals and medical centres.

Drugs that block the cellular consumption of fat for the treatment of metastasis
The Stem Cells and Cancer laboratory, headed by Dr. Salvador Aznar-Benitah at IRB Barcelona, has discovered that a protein called CD36 is decisive for the metastatic capacity of cancer cells.
CD36 is found on the membranes of tumour cells and is responsible for capturing and internalizing fatty acids. Using laboratory animal models, researchers have observed that when they block CD36, they can prevent the formation of metastases or drastically reduce those that are already formed. These results have been verified in different types of carcinomas (oral, breast, lung, ovary, bladder and melanoma), and they demonstrate that there is a direct relationship between the consumption of fats (specifically, palmitic acid) and the enhancement of metastasis through CD36.
As a result of these results, IRB Barcelona and the Institution for Research and Advanced Studies (ICREA) have founded the company Ona Therapeutics, which focuses on discovering drugs for the treatment of metastatic cancer.
Experimental therapies based on immunotherapy for the treatment of advanced colorectal cancer
Immunotherapy is a type of treatment that involves boosting the immune system to help the body eliminate cancer cells. This technique has quickly become a very effective weapon to treat some kinds of cancer, such as melanoma or lung cancer. However, most colon tumours do not respond to this type of treatment.
The Colorectal Cancer laboratory, led by Dr. Eduard Batlle, has discovered that the hormone TGF-beta is responsible for the immune system not recognizing or eliminating colon tumour cells. Using animal models, the research team has shown that the combination of an inhibitor that blocks TGF-beta with already available immunotherapies eliminates established metastases that would otherwise cause death.
Dr. Batlle's group has also identified for the first time the cells responsible for colorectal cancer reappearing in the form of metastases after the removal of the primary tumour. These residual tumour cells, which are hidden in the liver and lung, are not picked up by the detection methods currently available and can be eliminated through immunotherapy treatment before surgery.
These discoveries open avenues for the development of new therapies specifically aimed at eliminating residual or advanced disease, as well as new diagnostic tools to identify those patients at highest risk of relapse.
Targeted protein degradation as a therapeutic tool
The Targeted Protein Degradation and Drug Discovery group, led by Dr. Cristina Mayor- Ruiz, applies the technique of targeted protein removal (degradation) to the molecular study of cancer and the discovery of new therapeutic approaches.
Currently, around 80% of all the proteins in our body are beyond the reach of traditional drugs. Dr. Mayor-Ruiz's laboratory aims to systematize the discovery and generation of new drugs that degrade proteins that contribute to the multiplication of cancer cells, thus offering new treatment opportunities.

To support this new line of research, IRB Barcelona has launched a drug discovery unit (Drug Screening Platform), in a clear commitment to speed up the transfer of research results to clinical practice.
The laboratory headed by Dr. Cristina Mayor-Ruiz is a clear example of IRB Barcelona's new laboratories that are developing projects in the most promising areas of cancer research. This progress has been possible thanks to the trust of the people and entities that support us.
Download ‘Metastasis: questions and answers’ ebook
Would you like to receive our ebook of questions and answers about metastasis? Fill in the form below

Make your donation to the Metastasis Challenge
€4,884,357.99
Total amount raised
€5,000,000
Goal
How much tax relief do you get on your donation?
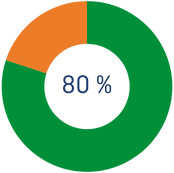
*For the first €250 you donate during the year, you will get 80% tax relief
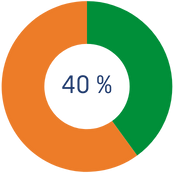
*You will get 40% tax relief on the amount exceeding €250
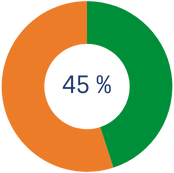
*From the 3rd year onwards, if you continue to donate an amount equal to or greater than the previous year to the IRB Barcelona, tax relief increases to 45% on the amount exceeding €250.
Testimonials
Patients
Other ways to join the challenge
Organize your own "Mission: Possible”
#MetastasisChallenge
Are you an athlete? Do you do handicrafts? Are you getting married? Is your company, school or club having a party?...and do you want to get involved in the fight against metastasis? Launch your own campaign here and help us go further. If you are still racking your brain to come up with an idea, take a look at other fundraising initiatives that are already up and running.
Latest news from the Metastasis Challenge
Contributors to the Metastasis Challenge