Images
- The study has focused on unravelling the exact function of the proteins GEMC1 and MCIDAS that activate multicilliogenesis.
- Impaired multiciliogenesis is associated with respiratory diseases, brain defects and infertility.
- The study has been published in the journal Cell Death & Disease, part of the Nature group.
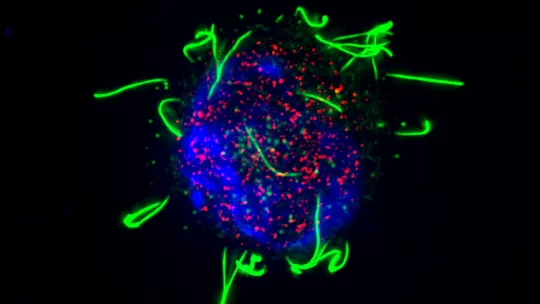
Multiciliated cells (MCC) are specialised epithelial cells that project between dozens to hundreds of motile cilia—cellular appendages that facilitate locomotor functions. Present in the respiratory tract, brain, and reproductive system, these cells are responsible for ensuring the correct circulation of cerebrospinal fluid in the brain, expelling mucus from the respiratory tract, and transporting the oocyte and spermatozoa in the oviduct and efferent ducts, respectively.
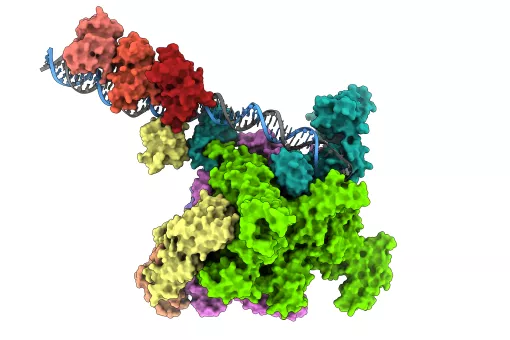
The cellular specialization of MCCs, also called multiciliogenesis, requires the atypical action of the transcription-activating proteins GEMC1 and MCIDAS, which enable the expression of the necessary genes. These proteins do not have identifiable DNA-binding sites but they both interact with the E2F4/5-DP1 transcription factors through a conserved C-terminal domain called the "TIRT domain." Although both proteins have a similar role in MCC differentiation, their activation mechanism and joint action are not entirely clear.
A study led by Dr. Travis Stracker, at IRB Barcelona, showed that GEMC1 and MCIDAS share similar interactomes but activate a distinct set of genes. It was identified that specific components of the SWI/SNF family, a protein complex involved in DNA organization, selectively collaborate with both proteins. Dr. Stracker is now a Principal Investigator at the Center for Cancer Research, National Cancer Institute, in the United States.
"Our data suggest that the differential action of distinct SWI/SNF subcomplexes interacting with GEMC1 and MCIDAS is necessary for MCC-specific transcriptional regulation, which is mediated by their distinct C-terminal domains," says Dr. Michael Lewis, first author of the study, who carried out the work as part of his doctoral studies.
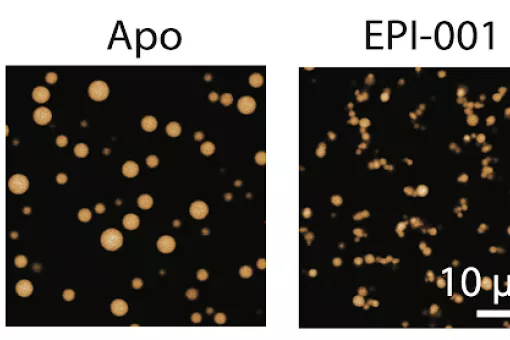
While GEMC1 interacts with the ARID1A-containing complex, MCIDAS interacts mainly with the BRD9-containing complex. When BRD9 protein is eliminated—through the use of PROTAC (chimeric molecules that destroy specific proteins)— in cancer cell models in vitro, multiciliogenesis is inhibited. These results were confirmed when MCC formation was prevented by inhibiting BRD9 protein in mouse cell models of multiciliogenesis.
A greater understanding of ciliopathies
Ciliopathies are a group of diseases with genetic causes that involve some type of deformation or function of cilia (singular: cilium), microtubule-based organelles that extend from the cell membrane. The symptoms of these diseases are usually varied, depending on the mutation, but all of them have in common a deficient function of this organelle, which, under normal conditions, plays an important and complex role in many vital cellular activities for the proper functioning of the organism.
Both GEMC1 and MCIDAS are regulators of a program to generate multiciliated cells and mutations in this pathway has been linked to patients with severe ciliopathies. These diseases include respiratory disorders, fertility problems, and hydrocephalus. This research is of special interest as it shed light on the biology of this specialised cell type (MCCs).
“Our work provides new information on the transcriptional machinery that GEMC1 and MCIDAS use in MCC differentiation and provides further resources for future research into the molecular functions of these transcriptional activators” explains Dr. Stracker.
Several lines of research can be explored from this mapping of interactions, some of which can potentially lead to the development of therapeutic approaches for specific ciliopathies.
The work has been carried out with the team headed by Dr. Xavier Salvatella, head of the Molecular Biophysics laboratory, also at IRB Barcelona, and other national and international collaborators.
Related article
GEMC1 and MCIDAS interactions with SWI/SNF complexes regulate the multiciliated cell-specific transcriptional program
Michael Lewis, Berta Terré, Philip A. Knobel, Tao Cheng, Hao Lu, Camille Stephan-Otto Attolini, Jordann Smak, Etienne Coyaud, Isabel Garcia-Cao, Shalu Sharma, Chithran Vineethakumari, Jessica Querol, Gabriel Gil-Gómez, Gabriele Piergiovanni, Vincenzo Costanzo, Sandra Peiró, Brian Raught, Haotian Zhao, Xavier Salvatella, Sudipto Roy, Moe R. Mahjoub & Travis H. Stracker
Cell Death & Disease (2023) DOI: 10.1038/s41419-023-05720-4
About IRB Barcelona
The Institute for Research in Biomedicine (IRB Barcelona) pursues a society free of disease. To this end, it conducts multidisciplinary research of excellence to cure cancer and other diseases linked to ageing. It establishes technology transfer agreements with the pharmaceutical industry and major hospitals to bring research results closer to society, and organises a range of science outreach activities to engage the public in an open dialogue. IRB Barcelona is an international centre that hosts 400 researchers and more than 30 nationalities. Recognised as a Severo Ochoa Centre of Excellence since 2011, IRB Barcelona is a CERCA centre and member of the Barcelona Institute of Science and Technology (BIST).