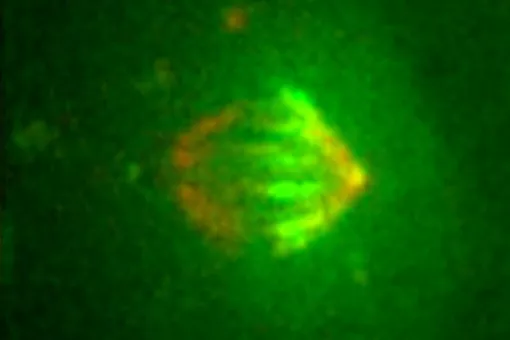
Advanced Digital Microscopy (ADM)
The Advanced Digital Microscopy core facility supports scientists from the IRB Barcelona community and external institutions/companies in performing advanced optical imaging and bioimage analysis, on their biological samples (S1 safety level, pre-clinical imaging, high-resolution imaging). The team supports users with training, project design, image acquisition and troubleshooting, custom automated acquisition, sample preparation, bioimage analysis tools and custom workflows, hardware-specific custom solutions, and more. The ADM is also committed to develop research instrumentation to adapt, improve or newly implement innovative biology-driven imaging applications, for example in the field of mesoscopic imaging (light-sheet-based fluorescence microscopy). The unit is accessible 24/7, users can book instruments autonomously and access analysis workstations onsite or remotely.
ADM is a facility of EUROBIOIMAGING-ERIC and provides services to external researchers within two of its Access Nodes: the Barcelona Mesoscopic Imaging Node, and the Barcelona Live and Intravital Imaging Node.

Services for IRB Barcelona and external researchers (Public or Private)
3D/4D/nD BIOIMAGING
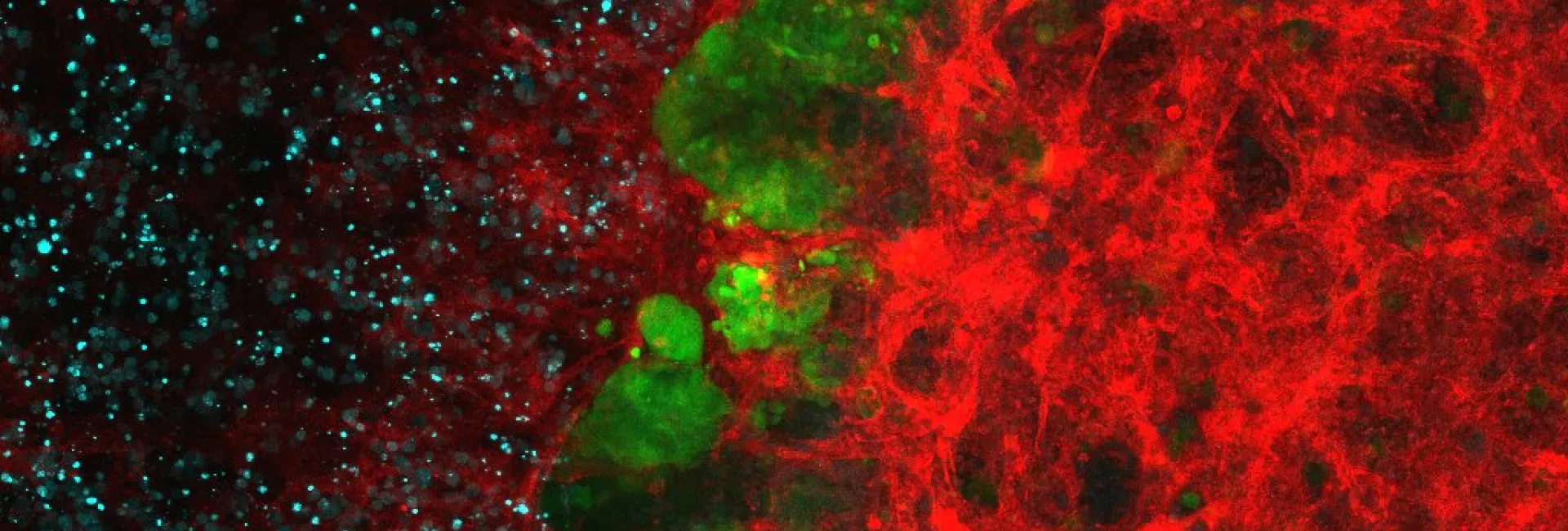
- Spectral laser scanning confocal microscopy. 3D, 4D and 5D imaging with optical sectioning, custom spectral detection and resolution, multiple position or high-content mode, and incubated environment control for living cells.
Four LSM confocal units: Inverted confocal systems (Stellaris white laser, LSM 780, LSM880) and one upright confocal system (Leica SPE). - Spinning disk confocal microscopy. Fast 5D imaging with the Andor Revolution system equipped with EMCCD camera technology, with FRAP-Photoactivation and microinjection, and with the CSU-W1 Yokogawa disk equipped with two sCMOS cameras, robot loader for High-Throughput.
NANOSCOPY
- Super Resolution. The Zeiss Elyra PS1 system combines confocal microscopy (LSM880) with three modalities of super resolution: Image Scanning Microscopy (aka Airyscan), Structured Illumination Microscopy (SIM), and Single Molecule Localization Microscopy (SMLM: STORM, PALM and variants).
- Combined STED with FLIM and white laser: The Stellaris system combines nanoscopy with STED (Stimulated Emission Depletion) for optical resolution down to 50 nm, Fluorescence Lifetime Imaging and flexible excitation with a white laser spanning the full visible range.
HIGH CONTENT IMAGING/SCREENING
The Olympus ScanR enables fully automated inverted microscope for imaging wide areas, multiwell plates and multipositions, automated live imaging with temperature incubation and CO2 control, superstable illumination for reproducible and high content image analysis, and Total internal reflection microscopy (TIRF) for high contrast imaging at surfaces.
The Nikon LIPSI system enables fast High-Throughput Screening imaging with confocal spinning disk modality (CSU-W1 from Yokogawa) and multiple multiwell plate readouts, thanks to the integrated and multi-sample incubator that loads samples automatically. HTS can be performed on up to 20 plates. The system is also very suited to very long-term imaging (1- to 2-week duration) by enabling the robot-driven loading of multiple experiments in parallel.
PHOTOMANIPULATION
Laser manipulation of living cells and organisms. FRAP and laser nanosurgery combined on a wide field fluorescence microscope to perform FRAP and fluorescence patterning, cell ablation, DNA damage, subcellular ablation and correlative microscopy. The Zeiss Axiovert 200M laser cutter is a custom platform equipped with a pulsed UV laser (355 nm, 470 ps) and spinning disk detection.
LIGHTSHEET MICROSCOPY / MESOSCOPIC IMAGING
- MacroSPIM for optically cleared organs: A double-sided macroscope-base detection and double-sided illumination lightsheet system for fluorescence and label-free 3D imaging of tissues/organs/organisms ranging from 0.5mm to 2.5cm.
- Optical clearing: we offer expertise with the most recent optical tissue clearing techniques, including BABB (Murray’s clear), CUBIC, DISCO family, ECI, DeepClear, and more.
- Lightsheet Microscope based on LEGO bricks, LEMOLISH an open source instrument enabling lightsheet imaging of cleared organs of size up to 5 cm.
- Lighsheet Microscopy for living organisms: A high-resolution double-sided illumination SPIM system, including multiview and sample rotation, for full volume live imaging, for samples ranging from 50 µm to 1 mm.
- High-throughput OPM. A highly automated custom Oblique Plane Microscope system for high-content imaging of organoids in multiwell plates, developed by Dunsby group (Imperial College) and part of the MACH3CANCER project, funded by the AECC.
BIOIMAGE ANALYSIS
Image processing and analysis is available from two workstations equipped with Imaris 9, customized ImageJ/Fiji bundles, NIS Elements, Matlab, CellProfiler, Machine Learning tools (e.g. CellPose), Ilastik, Napari, Voreen, Leica suites and more. Custom Image Analysis workflows are developed on demand, tailored to imaging projects. Open source projects developed by ADM are also available, e.g. LOBSTER for large image data, MosaicExporerJ for large lightsheet datasets stitching, AutoscanJ for intelligent Microscopy, and more.
Conventional transmission and fluorescence microscopy. Bright field, phase contrast, differential interference contrast (DIC), dark field and spectral fluorescence imaging of fixed samples, colour-based brightfield imaging (e.g. histological sections). Fluorescence stereoscopy/macroscopy for sample manipulation and dissection, extended focus imaging, live macroscopy. Available instruments for BF imaging: Stereoscope, fluorescence Macroscope (Olympus MVX10), inverted epifluorescence microscope (ScanR), LIPSI.

STED+FLIM with White Laser, Leica Microsystems.
Nanoscopy with SIM/dSTORM/Airyscan, Carl Zeiss Microscopy.

Hyperspectral confocal, Carl Zeiss Microscopy.
Confocal with robot loader, Nikon Instruments Inc.
Widefield automated imaging + TIRF, Olympus (Evident).

Spinning disk microscopy with FRAP/PA, Andor (Oxford Instruments).
Macroscope w. fluorescence, Olympus (Evident).

Laser scanning confocal, Leica Microsystems
Custom instrument, lightsheet microscopy/mesoscopic imaging.

Oblique Plane Microscope, Long-term high-throughput lightsheet imaging.
Custom instrument for live laser ablation
- Request consultation or training: each instrument requires a specific training, new access to instruments requires prior discussion and analysis of the needs and specifications (technology, sample preparation, expected image analysis needs). Contact the team through microscopy@irbbarcelona.org to arrange an appointment.
- Instrument booking: the user books the equipment for a specific time slot in AGENDO. New users contact microscopy@irbbarcelona.org. to gain access. Some instruments may require prior approval or training.
- Usage: the user operates the equipment following facility guidelines.
- Data acquisition
- After use: the user follows shutdown and/or cleaning procedures.
- Issues report: during office hours, please come to see us for fast problem solving, otherwise contact microscopy@irbbarcelona.org.
This CRUK Accelerator project aims to explore the trade-off between complexity of 3D cancer models and power of assays (in terms of single cell resolution and throughput) by developing modular, open source, automated instrumentation for rapid 3D imaging of such complex 3D cancer models and testing these instruments on a range of 3D cell cultures with different biological and optical properties. The emphasis on open source instrumentation is intended to enable other laboratories to replicate the capabilities that we develop in this Accelerator. The partners explore and optimise cell culture and sample preparation (e.g. labelling, mounting, clearing) techniques and share our know-how to enable researchers to optimise 3D assays to address their specific cancer biology questions. At IRB Barcelona, MACH3CANCER objectives are unravelled by the Batlle’s laboratory and the ADM facility. The project has brought the installation of the first OPM (Oblique Plane Microscope) in Spain.